
GIBBERELLIC ACID
MOLECULAR STRUCTURE:

IUPAC SYSTEMATIC NAME:
2,4a,7-Trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10-dicarboxylic acid 1,4a-lactone
CAS REGISTRY #:
77-06-5
MOLECULAR FORMULA:
C19H22O6
MOLECULAR WEIGHT:
346.38 g/mol
% COMPOSITION:
C 65.9%, H 6.4%, O 27.7%
MELTING POINT:
233 - 235 C
SPECIFIC ROTATION (in ETOH):
[ a ]19D +86
PHYSICAL APPEARANCE:
White powder
INFRARED SPECTROSCOPY (NUJOL):

MASS SPECTRUM:

13CNMR (50.18 MHz, DMSO-d6):

1HNMR (399.65 MHz, DMSO-d6):


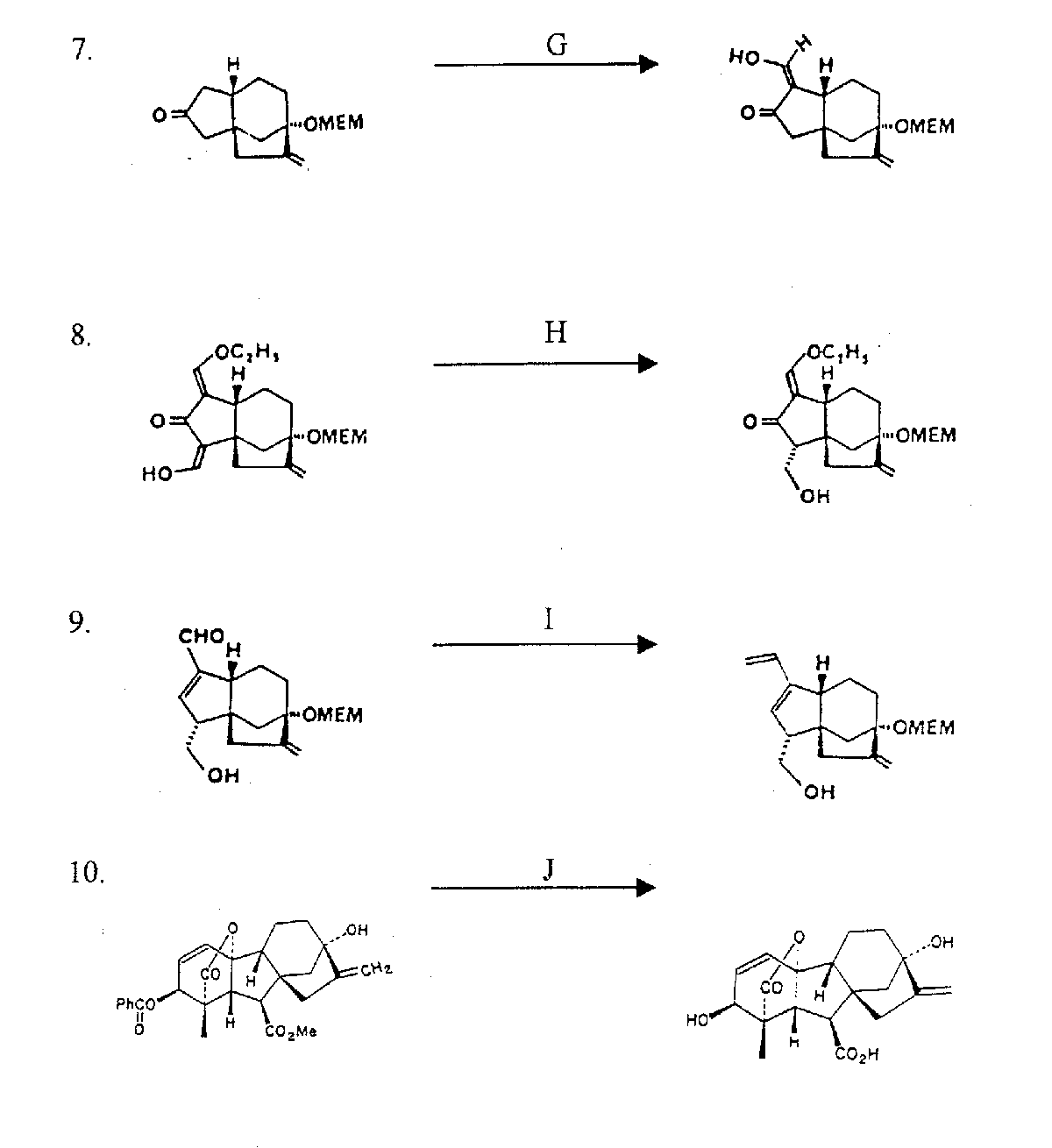
LEGEND:
A = 1.5 equivalents each of diethyl 2-oxo-propylphosphate and potassium hydroxide in 4:1 ethanol-water at 5 C for 28 hours
B = 2 equivalents of divinyl cuprate in THF at -50 C for 15 minutes
C = 6:1 ethylene glycol-triethyl orthoformate and p-toleune sulfonic acid (~0.075 equivalents) at 590C for 1.5 hours
D = 3 equivalents of disiamylborane in THF at 250C for 17 hours
E = 20 equivalents of NaOH in ethanol at 00C for 2.5 hours
F = 5 equivalents of methylenetriphenylphosphorane in 2.5:1 THF-HMPA for 1.8 hours
(OMEM = methoxyethoxymethoxy ether)
G = 6 equivalents of NaOH, 30 equivalents ethylformate, and a trace of ethanol in 1,2-dimethoxyethane for 1 hour at 0-250C
H = 5 equivalents NaH in THF at 250C, 5 equivalents of sodium bis(2-methoxyethoxy)aluminum hydride at -20 - 00C for 50 minutes, and quenching with ammonium chloride at 00C
I = 10 equivalents of methylenetriphenylphosphorane in THF at 00C for 10 minutes
J = hydrolysis at pH 10 with K2CO3/KHCO3, MeOH/THF/H2O (4:1:1) at 250C for 1 hour
DISCUSSION:
Gibberellic acid, or GA3, is a diterpenoid acid that functions as a plant growth-regulating hormone. It is one of more than 90 compounds in a group of compounds named gibberellins. They affect many mechanisms of plant growth including cell elongation, cell division, flowering, fruit development, breaking dormancy, and more. The origin of research into gibberellins can be traced back to Japanese plant pathologists in the late 1800's.
At this time plant pathologists were investigating a plant disease referred to as "bakanae" (foolish seedling) which drastically lowered the yields of rice throughout the Asian continent. Symptoms of the disease included exceptionally tall seedlings that toppled over before they had a chance to mature and flower, pale yellow seedlings, slender leaves, and stunted roots. The first paper on the cause of bakanae was published in 1898 by Shotaro Hori. He tried to show that the disease was induced by a fungus that belonged to the genus Fusarium. Later, in 1912, Sawada published a paper in the Formosan Agricultural Review entitled "The Diseases of Crops in Taiwan" where he also tied the elongation of rice seedlings affected by bakanae to a stimulus derived from fungal hyphae. Then, in 1926, a Japanese scientist named Eiichi Kurosawa discovered that the disease was actually caused from a fungus of the genus Gibberella. His work with cultured filtrates from dried rice seedlings led him to his discovery and he found that the chemical secreted by the fungus stimulated shoot elongation, inhibited chlorophyll formation, and suppressed root growth.
It wasn't until 1934 that the first gibberellin was isolated. Teijiro Yabuto initiated the work and he is responsible for the first use of the term "gibberellin". Western scientists didn't learn of gibberellins until after World War II, and before the mid-1950's scientists didn't realize that many gibberellins occur naturally in higher plants. The first proof of this was done by Margaret Radley at Akers Research Laboratories in the UK. As technology improved more and more gibberellins were identified. Gibberellic acid, identified around 1950, was one of the first gibberellins to be isolated. Currently there are 94 known gibberellins.
All the gibberellins have a common molecular theme, such as a five- ring system, but they have subtle variations. These variations enable some forms to be more active than others in plants. The knowledge of gibberellins and how they work has been very important to agriculture. Today, bakanae is easily prevented by treatment of seeds with fungicides before sowing. Gibberellins, although structurally complex with bicyclic rings and 8 stereocenters, are now synthesized for their use in agriculture. They affect plants in many ways that farmers can use to their benefit.
In plants themselves, gibberellins are mainly produced in roots and young leaves, however they affect growth in leaves and stems, and have little to no effect on roots. In stems, gibberellins stimulate cell elongation and mitosis. They act synergistically with other plant hormones named auxins in a manner that is not yet understood. This role in stem elongation can especially be seen when gibberellins are applied to dwarf plant species. These species, which for some reason lack their own gibberellins, respond quite well to applied gibberellins and proceed to grow to normal heights. Normal plants do not respond because apparently they already produce their own optimal dose of the hormone. Gibberellins are also responsible for bolting, which is the growth of a floral stalk. Some plants stay low to the ground with very short internodes in their nonflowering stage. When a gibberellin is produced the plant switches to a stage of reproductive growth as its stems elongate and elevate the flowers that are developing at the tips of the stems. Gibberellins also act with auxins in fruit development. In some plants both hormones must be present for fruit to set. The use of gibberellins in the spraying of Thompson seedless grapes is perhaps the most important commercial gibberellins application concerning fruits. It causes the grapes to grow larger and further apart, which consumers prefer.
Finally, one of the most important functions of gibberellins overall is in breaking dormancy and initiating germination. Many seeds have a high concentration of gibberellins, especially in the embryo. When water is let into the seed the release of gibberellins from the embryo signals the seed to break dormancy and germinate. Many seeds that require specific environmental conditions, such as exposure to light or cold temperatures, will break dormancy when they are treated with gibberellins. Also, gibberellins stimulate the synthesis of some digestive hormones, such as a -amylase, in some plants. They do this specifically by stimulating the synthesis of the messenger RNA that codes for the enzyme. In this way, gibberellins affect gene expression. With all this knowledge of how gibberellins behave, people in agriculture have been able to use them to our advantage. Some of the most common commercial products are Activol, Berelex, Cekugib, Gibberellin, Gibrel, Pro-Gibb, Pro-Gibb Plus, and Regulex.
With all these positive aspects of gibberellins known, one might begin to wonder if there is any risk involved in their use. The answer ultimately is no. Experimental animals have tolerated large doses of gibberellins without apparent adverse effects, and no human poisonings have been reported. They only cause minor irritations when they come in contact with skin, and this can easily be avoided by the use of gloves. Gibberellins are not listed as carcinogenic or hazardous by any major health organizations such as OSHA, ACGIH, IARC, NIOSH, and NTP. With this in mind, it seems that gibberellins and their discovery have been quite beneficial to farming industries, and therefore beneficial to mankind as a whole.
BIBLIOGRAPHY:
Cambell, Neil A. "Gibberellins." Biology 4th Edition, p.757-758. Menlo Park, CA: The Benjamin/Cummings Publishing Company, Inc.
Corey, E.J., et al. "Total Synthesis of Gibberellic Acid: A New and Effective Route to
A Key Tricyclic Intermediate." Journal of American Chemical Society 1979 Vol. 101(4), p.1038-1039
Croker, Steve. "Gibberellins: A Short History." IACR-Long Ashton World Wide Web:
www./ars.bbsrc.ac.uk/plantsci/gas.html
Gibberellic Acid. Vermont SIRI Material Safety Data Sheets
World Wide Web:siri.org/msds/index.html
Krishnamoorthy, H N (1975). Gibberellins and Plant Growth, p. 20. New York: John Wiley & Sons.
Lombardo, Luciano, et al. "General Strategy for Gibberellin Synthesis." Journal of American Chemical Society 1980 Vol. 102, p.6626-6628
Pouchert, Charles J. The Aldrich Library of Infrared Spectra 2nd Edition. p. 363f
Spectral Data Base System for Organic Compounds. World Wide Web:
www.aist.go.jp/RIODB/SDBS/perl/img_disp
Voigt, D. et al. Organic Mass Spectroscopy , Vol. 13, No.10, 1978 p.600