Temperature
The
SI unit of temperature is Kelvin. It is usually referred to as the
thermodynamic temperature and is used primarily in scientific applications. The
Kelvin scale begins at 0, which is referred to as “absolute zero”, that is,
there are no negative values of Kelvin unlike Fahrenheit and Celsius, which is
more commonly used throughout the world.
http://physics.nist.gov/cuu/Units/kelvin.html
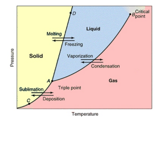 The definition of the unit of thermodynamic temperature
was given in 1954, which selected the triple point of water as the fundamental
fixed point and assigned to it the temperature 273.16 K, and in so doing
defined the unit. (See the Phase Diagram on the right. Consider that at the
precise temperature and pressure where the “triple point” exists all three
forms (phases) of water are in equilibrium at the same time. The
name Kelvin (symbol K) instead of "degree Kelvin, (symbol
°K)" was adopted in 1967 and defined the unit of thermodynamic temperature
as follows: The Kelvin, unit of
thermodynamic temperature, is the fraction 1/273.16 of the thermodynamic
temperature of the triple point of water.
The definition of the unit of thermodynamic temperature
was given in 1954, which selected the triple point of water as the fundamental
fixed point and assigned to it the temperature 273.16 K, and in so doing
defined the unit. (See the Phase Diagram on the right. Consider that at the
precise temperature and pressure where the “triple point” exists all three
forms (phases) of water are in equilibrium at the same time. The
name Kelvin (symbol K) instead of "degree Kelvin, (symbol
°K)" was adopted in 1967 and defined the unit of thermodynamic temperature
as follows: The Kelvin, unit of
thermodynamic temperature, is the fraction 1/273.16 of the thermodynamic
temperature of the triple point of water.
Because of the way temperature scales used to be defined,
it remains common practice to express thermodynamic temperature, T, in
terms of its difference from the reference temperature To =
273.15 K, the freezing/melting point. This temperature difference is called a
Celsius temperature (symbol °C), t in the equation below, and is defined by the
equation
t= T- To
t (oC)= T(K)
- 273.15K
The unit of Celsius temperature
is the degree Celsius, symbol °C, which is by definition equal in magnitude to
the Kelvin. A difference in temperature at two different conditions may be
expressed in Kelvins or in degrees Celsius. These are equal to each other.
ΔT = Tfinal
- Tinitial = Δt = tfinal
- tinitial
The numerical value of a Celsius
temperature t expressed in degrees Celsius is given by
t/°C = T/K - 273.15.
In 1989/1990 the Kelvin and the
degree Celsius were adopted as the International Temperature Scale.