Before beginning this exercise complete the Chime tutorial:
http://c4.cabrillo.cc.ca.us/projects/chime_tutorial/index.htm
Sources & Sinks of Atmospheric CO2
Uptake of Atmospheric CO2 and Energy
Sun light is absorbed in plants, which use an enzyme, chlorophyll, as
a catalyst to synthesize sugars, carbohydrates and starches. These carbon
compounds have the sun's energy converted into chemical bonds which essentially
store and provide the energy in various food sources used by man and animals.
Without plants, chlorophyll and the sun, life as we know it would be unable
to survive.
The images below illustrate photosynthesis of one sugar of the many
possibilities, This one is a form of glucose. The multi-colored image in
the center frame above the arrow is a structural rendering of a chlorophyll
molecule. Only the molecular "backbone" of chlorophyll is shown since this
particular protein has over 3600 indivdual atoms.
A) Write a complete, balanced equation with molecular formulas
for the photosynthesis of the above sugar.
B) How many liters of CO2 (g) will be needed to produce
1 pound (454 g) of glucose? (Treat CO2 as an ideal gas. Pressure
= 1 atm, Temperature = 25 oC) Show your calculations.
Combustion of Hydrocarbons / Atmospheric CO2 (g)
Plant and animal carbonaceous materials have been converted to "crude
oil" through microbial action and geochemical forces working over millions
of years. The hydrocarbon "crude oil" that is pumped from underground and
undersea deposits is processed into fuels ("fossil fuels") which are burned
to release chemical energy for manufacturing, transportation and conversion
into electricity.
The reaction below is the burning of isooctane, a key component of gasoline.
A) Write a complete, balanced equation with molecular formulas
for the combustion of isooctane.
B) How many liters of CO2 (g) are produced from the
combustion of 1 gallon of isooctane? (Isooctane density = 0.7 g/mL. Treat
CO2 as an ideal gas. Pressure = 1 atm, Exhaust temperature =
150oC) Show your calculations.
C) Global CO2 (g) Calculations:
1) What is the mass of CO2 produced per gallon
of gasoline? This amount is added to the CO2 already present
in the natural carbon cycle as additional CO2 (g) every year.
Ice cores in Antarctica have shown an increase of tropospheric CO2
(g) from about 280 ppm to 340 ppm (volume:volume) from 1800 to 1996.
http://cdiac.ESD.ORNL.GOV/trends/co2/graphics/lawdome.gif
http://cdiac.ESD.ORNL.GOV/trends/co2/lawdome.html
The troposphere is the atmosphere averaged to a height of 9 miles. Calculate
the total mass and volume of CO2 that has been added to the
troposphere since 1800. For the gas calculation assume a nominal pressure
of 600 torr and nominal temperature of 260 K for the troposphere. Show
your calculations.
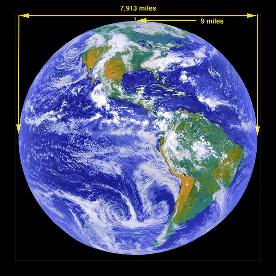
Image above: The earth from a GOES
/ NASA / NOAA satelite photo. GOES (Geostationary Operational Environmental
Satellites). http://rsd.gsfc.nasa.gov/goes/
2) a) An estimated 5 billion metric tons of CO2
is
added to the atmosphere each year. Assume that CO2 is an Ideal
gas at 75 oF and 1 atm. Calculate the volume of CO2 (g)
that
is added each year. b) If the atmosphere were a closed system and
the temperature increases by +2 oC while the volume is constant
(Use the volume calculated in 1) with 75 oF and 1 atm
as the initial temperature and initial pressure.), what is the effect on
the atmospheric pressure? Show your calculations.
3) Answer ques. #12 & #13 pp. 31, "What Should We Do About
Global Warming?"
4) Follow the graphing instructions and answer the questions:
http://www.athena.ivv.nasa.gov/curric/land/global/plotco2.html
5) Answer ques. #11 & #12 pp. 35-38, "What Should We Do
About Global Warming?"