KHP Post Lab: Titration Curves
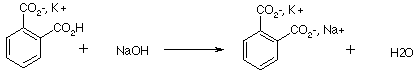
Select a partner. On graph paper, clearly and neatly plot the pH versus the volume of base using the following student data for the titration of a 2.4501g sample of impure KHP dissolved in about 50 mL of de-ionized water with 0.01000M NaOH. Turn in one plot (titration curve) per group with the names of both group members including answers to questions a-e with the work showing the mathematical solutions for questions c, d, and e.
|
|
|
|
Buret reading (mL) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
a) What is the pH at the equivalence point?
b) What volume of NaOH neutralized the KHP?
c) How many moles of KHP are present in the sample?
d) How many grams of KHP are present in the sample?
e) What is the percent KHP in the original sample?