 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
The chemistry of carbon is critical to providing energy to living organisms in many, many ways. One example is photosynthesis and another is combustion. In this exercise you will explore the thermodynamics of these two reactions in addition to a calculation that approximates the energy needed for maintaining the general metabolic levels that our bodies require.I) PhotosynthesisThermodynamic Data: NIST Chemistry WebBook
http://webbook.nist.gov/Thermochemical data for over 7000 organic and small inorganic compounds:
* Enthalpy of formation
* Enthalpy of combustion
* Heat capacity
* Entropy
* Phase transition enthalpies and temperatures
* Vapor pressure
Reaction thermochemistry data for over 8000 reactions.
* Enthalpy of reaction
* Free energy of reaction
Sunlight is absorbed in plants, which use an enzyme, chlorophyll, as a catalyst to synthesize sugars, carbohydrates and starches. These carbon compounds have the sun's energy converted into chemical bonds which essentially store and provide the energy in various food sources used by man and animals. Without plants, chlorophyll and the sun, life as we know it would be unable to survive.
A) Write a complete, balanced equation with molecular formulas for the photosynthesis of glucose(solid) from CO2 and water. The molecular formula for glucose can be found using the NIST link or ChemFinder . The heat of formation of glucose can be found from its molecular formula in published Thermodynamic Tables. Check your the table in your textbook.
B) How many Joules of energy are stored in 1 pound (454 g) of glucose? (Assume: Pressure = 1 atm, Temperature = 25 oC) Show your calculations.
II) Combustion
Plant and animal carbonaceous materials have been converted to "crude oil" through microbial action and geochemical forces working over millions of years. The hydrocarbon "crude oil" that is pumped from underground and undersea deposits is processed into fuels ("fossil fuels") which are burned to release chemical energy for manufacturing, transportation and conversion into electricity.
A) Write a complete, balanced equation with molecular formulas for the combustion of the light petroleum distillate, naphtha. Assume naphtha is 100% 2-methylpentane. The molecular formula for 2-methylpentane can be found using the NIST link or ChemFinder .
B) How many Joules are produced from the combustion of 1 gallon of naphtha? ( Density = 0.64 g/mL. Pressure = 1 atm, Temperature = 25 oC) Show your calculations.
C) How many liters of water can be boiled with the energy produced from burning 1 gallon of naphtha? Assume the water is at 25 oC. Show your calculations. You will need to find the specific heat of water.
III) Metabolism
The sum of the chemistry of our life processes is referred to as metabolism. This chemistry requires energy and our elevated body temperature is an affect of it.
Our bodies are 70% water by mass.
A) Calculate the amount of energy needed to raise the temperature of the mass of water in the body of you and your partner for the thermochemistry experiment from room temperature to its actual, hopefully "normal" temperature. Show your calculations.
Student A
Student BTotal Mass
kilograms_ _ Mass of H2O
kilograms_ _ Body
Temperature_ _ Room
Temperature_ _ B) Combustion of how many grams of glucose will you and your partner require to maintain body temperature plus an additional 10% for the intellectual energy needed to complete this exercise? Show your calculations.
C) How many Calories would this amount to? How many grams of protein is this equivalent to? How many grams of fat is this equivalent to?
D) From the chart below, estimate how many Calories you individually use per week. Are you getting enough Calories or too many?
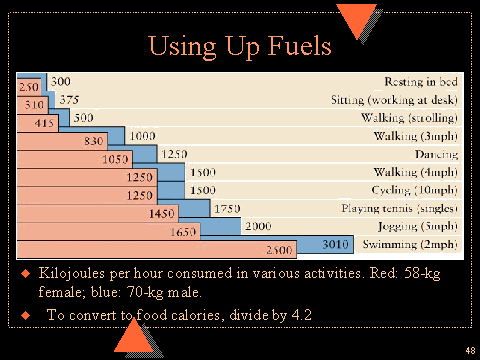
Lucille B. Garmon: http://www.westga.edu/~chem/courses/chem121s/121_06/sld048.htm
© Copyright 1998-2008 R.J. Rusay