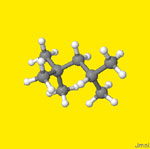
A) Organize the 12 compounds into two groups: an acyclic group (6 compounds)
and a cyclic group (6 compounds). Enter line structures for each
in the table below; beginning
with the largest ring structure for the cyclic and the least substituted
for the acyclic. Add the respective combustion data for each compound to
the table using data for combustion in either the condensed phase (liquid)
or the gas phase DcH from the NIST WebBook.
Draw energy diagrams which illustrates the combustion data for each of
the cyclic and acyclic compounds. (Separate those that you compare in the
gas phase from those in the condensed phase, and compare separately.)
B) For the cyclic group (C) rank the compounds in order of stability from
highest to lowest.:
Gas phase:
____ > ____ > ____ > ____ > ____ > ____
Condensed phase:
____ > ____ > ____ > ____ > ____ > ____
C) For the acyclic group (A) rank the compounds
in order of stability from highest
to lowest.:
Gas phase:
____ > ____ > ____ > ____ > ____ > ____
Condensed phase:
____ > ____ > ____ > ____ > ____ > ____
D)
Analyze the data looking for patterns and trends. Explain any trends and what
structural features could contribute to differences between the structures
of the isomers and the energy produced from burning each of them.
E) From the data are cis or trans isomers more stable? Briefly explain
why from a theoretical, molecular basis.
F) From the data are larger rings more or less stable? Briefly explain
why from a theoretical, molecular basis.