Pre lab Questions
A) Refer to the gas chromatograph (below) of a mixture of Compound
A (RT = 1.11) and Compound B (RT=2.27), which was done on a general purpose column:
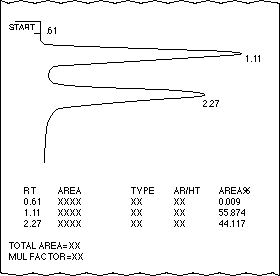
- Which compound has the highest retention time?
- Which compound is present in a larger amount assuming that their mass correction
factors are equal?
- Which compound has the lower boiling point?
- What would happen to the retention times of compounds A and B if the column
temperature were raised?
For questions B and C consult: http://chemconnections.llnl.gov/Organic/Chem226/Labs/GC/LiqPhases.htm
B) Consider the following compounds:
| compound |
boiling point
|
| A) methyl cyclohexane |
101
|
| B) pentane |
36
|
| C) octane |
126
|
| D) 2,3-dimethyl octane |
165
|
| E) heptane |
98
|
If the compounds in the table above are run on a GC column that has DC-710 as
the stationary phase,
rank the compounds in the order that they would be detected coming off of the
column.
C) Consider the following compounds:
| compound |
boiling point |
| A) propionic acid |
141
|
| B) 2-hexanol |
140
|
| C) isoamyl acetate |
142
|
| D) 3,4-dimethylheptane |
140
|
If these compounds are run on a GC column that has DEGS as
the stationary phase, rank the compounds in increasing
order of retention times.
D) You think that you injected your sample into the GC,
but
you see no peak after five minutes. So, you increase the data sampling period
for another
five minutes, but see nothing.
You repeat this two more times but still see nothing.What
should
you
do?
(Ask Dr. R. is NOT a correct response.)
E) State the stationary and moving (mobile) phases in each type of chromatography
below:
-
gas (GC)
- thin layer (TLC)
F) Rank the following compounds in terms of their expected retention times
on a GC column that has OV-101, a non polar material, as
the stationary phase, and then rank them according to predicted order of Rf values
on
a
silica
gel
TLC
plate
that is eluted with hexane as the solvent.
(b.p. and m.p. = oC; d = g/mL)
I........................ II ..........................III
......................IV



