Organic reactions can be described by one or more of the following terms: non-regioselective, regioselective, non-stereoselective, stereoselective, stereospecific.
The three reaction mechanisms: carbocation, free-radical, and concerted, which you have been introduced to thus far, contribute to the reaction's designation above. In order to decide which type of reaction occurs, it is necessary to compare the reactant(s) and product(s), tracking new bonds that have been made and the stereochemistry of the carbon atoms that have new bonds added to them.
A simple carbocation or free radical reaction mechanism are generally non-stereoselective. However, both of these reactions are regioselective, favoring one type of substituted carbon atom over another, and either following Markovnikov's Rule or not (Anti-Markovnikov) respectively. Other reactions such as bromination of an alkene are stereospecific in bond formation, mechanism, and manner of addition. Several addition reactions that you have been introduced to add electrophilically to a carbon-carbon double bond either from the same side of the C=C double bond (syn addition) or from opposite sides (anti addition). Bromination is regarded to add bromine atoms to the double bond by way of anti addition through a bromonium ion intermediate.
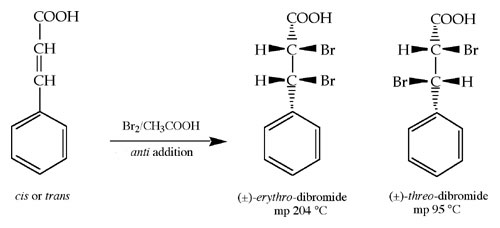
This experiment is to consider the established anti addition mechanism of bromine to identify the unknown stereochemistry of a starting cinnamic acid stereoisomer. Your unknown may be either the cis or trans stereoisomer. Analysis of the product's melting point will allow you to determine which stereoisomer was started with.
Procedure: (Budget 1.0 lab period + 1/2 lab period for sample prep m.p.)
|
Weigh your cinnamic acid unknown sample into a 50 mL Erlenmeyer flask and
add 6.0 mL of glacial acetic acid. Take your separatory funnel to the hood,
put 8.2
mL of a 1.25 M solution of bromine in acetic acid into the funnel and stopper
it immediately. Back in your hood, securely support the separatory funnel
over the flask.
You must add the bromine/acetic acid solution in five or more portions. Add
a portion, swirl the flask, and wait until the color has faded to light orange
before adding the next portion. The cinnamic acid should dissolve shortly
after the addition of the first portion, and the entire addition should take
about half an hour.
After the last addition, let the reaction mixture stand at room temperature
for 15 minutes with occasional swirling. If the mixture becomes colorless
during this period, add more of the bromine/acetic acid mixture until the
yellow color just persists. If the mixture has a distinct yellow or orange
color at the end of the reaction period, add cyclohexene until it is colorless
or nearly so.
Cool the flask in ice water until crystallization is complete, then collect
the product by vacuum filtration. Leave the solid in the Buchner funnel
and run cold water through it until the acetic acid smell is hardly noticeable.
Dry the product and measure its mass and melting point.
The hot solution is cooled in an ice-water bath until precipitation appears
to have ceased. The recrystallized salicylic acid is vacuum filtered,
air dried for a few minutes, and then left in an open container to dry
until
the next lab period. At that time, you can weigh your dry sample and
calculate your % yield. Record the melting point .
Report in the results section: your unknown number, which diastereomeric
pair formed: erythro or threo, and
which stereoisomer of cinnamic acid you began with, cis or trans.
Post lab Questions:
1) Hypothetically, a student observed that the optical rotation
measured for the products at the completion of the bromination of cinnamic
acid was 0o.
a) Explain this result by showing stereochemical structures for
the two possible products that would support the data.
b)
How much of each of the products would be present
in the product mixture (percent wise)?
Briefly explain your answer.
c) Illustrate
how the two products could be separated by chemical means. (Use
chemical equations and Fisher drawings for the reactants and products; (S)-(-)α-phenethylamine
is to be used as the resolving agent in your reactions.
If you require a base or acid, you have 2M solutions of NaOH and H2SO4 available.
d) If the student began with 2.00 grams of a 50:50 product mixture, and wound
up
with
750mg of recrystallized product that rotated polarized light counter-clockwise,
what is the overall % yield of the l-stereoisomer? Show your calculation.
2) Draw structures which illustrate the mechanism and stereochemistry of bromination of cis-stilbene.
3) Draw a Fisher drawing of the bromination product of trans-stilbene. Identify the product as being either threo, erythro, or meso.
4) Briefly explain what physical or chemical properties
of a bromination product of stilbene, which is of unknown stereochemistry,
could be used to
determine if the starting stilbene were cis or trans. Cite actual reference
data for comparison if possible.