Introduction:
(Read Lab Text/Guide pp.116-125)
Separation of a mixture of chemical compounds and isolation of the individual compounds is very important. This can be achieved by a variety of methods. The most common method in organic chemistry is extraction, which depends on either a difference in either the physical and/or chemical properties of the componenets. If the compounds differ in their solubility, they can be separated by virtue of their different solubilities in a pair of immiscible solvents. An example of such a mixture would be sodium chloride and cholesterol, both of which are white, crystalline solids. Sodium chloride, an ionic salt, is soluble in water, but insoluble in the relatively non-polar, immiscible solvent ether, whereas cholesterol, a relatively neutral, non-polar organic molecule, is soluble in ether and only very sparingly soluble in water. Shaking the mixture with a mixture of water and ether would result in the formation of two immiscible solutions, one of sodium chloride in water and one of cholesterol in ether. Separation of the two solutions in a separatory funnel and the removal of the solvent by evaporation of each solution separately then leads to the respective isolation and recovery of the individual compounds.
In a case where there is not a distinct difference, such as the separation of sodium chloride from 3-aminobenzoic acid where one component (in this case the 3-aminobenzoic acid) is soluble in both water and ether, a process known as extraction must be used. In this case, the mixture is dissolved in water and the resulting solution shaken with ether in a separatory funnel. The 3-aminobenzoic acid, being soluble in both the aqueous and ethereal layers, will partition itself between the two layers and this partitioning is described by the partition or distribution coefficient, Kp, as follows:
Kp = solubility in ether / solubility in water
The solubility of 3-aminobenzoic acid in water at 20oC is 6.0 gram/litre (g/L), while in ether it is 20.0 g/L. Therefore,
Kp = (20 g/L) / (6 g/L) = 3.33
Suppose that 2.0 g of 3-aminobenzoic acid is present in the mixture and that the compound is allowed to partition itself between l litre of water and l litre of ether at 20 oC. How much of the acid will be present in each layer?
If Z is allowed to represent the concentration in g/mL of acid in the water, then from the definition of the partition coefficient,
Kp = (concentration of acid in ether) / Z = 3.33,
Thus, (concentration of acid in ether) = 3.33 x Z
wt. of acid in 1 L of water = l000 x Z
wt. of acid in 1 L of ether = l000 x 3.33 x Z
But,
total wt. of acid = 2.0 g
Therefore,
(l000 x Z) + (3.33 x l000 x Z) = 2.0 g
i.e. 4330 x Z = 2.0 g
or Z = 2.0 / 4330 = 0.000462 g/L
Therefore,
wt. of acid in water = 1000 x Z = 0.462 g
and
wt. of acid in ether = 1000 x 3.33 x Z = 1.538 g
Thus, by a single extraction of 1000 mL of aqueous solution by 1000 mL of ether, 1.538 g of the original 2 g of acid present in the water will be transferred to the ether layer. It should now be evident that by removal of the first litre of ether and re-extraction of the aqueous layer with a fresh batch of ether, partitioning of the remaining 0.462 g of acid between the aqueous and the second ethereal layers will result in the transfer of a further 0.355 g of acid into the ether, leaving only 0.107 g in the aqueous layer. The repeated extraction of the aqueous layer with fresh batches of ether will lead therefore to the removal of most of the acid into the ether. The acid may then be recovered by evaporation of the combined ether extracts.
In many cases, mixtures of organic compounds can be separated by methods which depend on a difference in their chemical properties such as behavior as an acid or base. Frequently, one or more compounds of a mixture is transformed into a water soluble salt for easy separation. Salts, in general, are water soluble and insoluble in organic solvents. For example, the acidic compound (HA):
Or the base (B:):
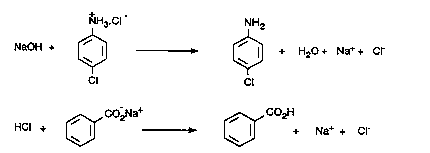
In this experiment, you will separate a mixture of 4-chloroaniline (a weak base), benzoic acid (a weak acid) and 4-dibromobenzene (a neutral compound), which illustrates this general process. This mixture can be readily separated since 4-chloroaniline reacts with HCl in water, benzoic acid reacts with sodium bicarbonate (NaHCO3) in water, and 4-dibromobenzene is insoluble in water. Thus, if a solution of a mixture of 4-dibromobenzene, 4-chloroaniline, and benzoic acid in ether is shaken with aqueous HCl the amine will be removed into the aqueous layer as its ammonium salt, whereas 4-dibromobenzene and benzoic acid will remain dissolved in the ether. The benzoic acid then can be extracted as its sodium salt by extracting the ether layer with aqueous NaHCO3.

After separation of the aqueous and etheral layers, the 4-chloroaniline can be regenerated by basification with aqueous NaOH, while benzoic acid can be regenerated from its sodium salt by acidification with dilute acid.
Procedure: (Budget 1.5 lab periods)
Weigh your unknown mixture of benzoic acid, 4-chloroaniline and 4-dibromobenzene, record an exact mass, and transfer the solid to a 125 mL Erlenmeyer flask. Add 10 - 15 mL of diethylether to the flask and swirl until the solid is completely dissolved. Transfer the solution to the separatory funnel in your Kem Kit, washing out the Erlenmeyer flask with a small volume (about 3-5 mL) of ether (use a Pasteur pipette) to ensure that no solid remains in the flask, and then add carefully to a separatory funnel 15 mL of 2 M HCl. Stopper the funnel and shake it carefully, remembering to release the pressure caused by the heat of the reaction at frequent intervals through the tap. When shaking is complete, remove the stopper and set the funnel on a ring clamp support ring, to allow the two layers to separate.
[Note: Ether is volatile and highly flammable. It has a boiling point (bp) of 35oC and a low flash point; during hot weather much of your ether may evaporate during the extraction process. If this occurs (as you will see by your upper layer being very small in volume) simply add 5 -10 mL more ether.]
When separation is complete, carefully run off the lower aqueous layer into a clean 125 mL Erlenmeyer flask, and then add a further 15 mL (approx.) of 2 M HCl to the remaining ether solution in the separatory funnel and repeat the extraction procedure. The second 15 mL of aqueous solution may then be run off and combined with 15 mL obtained from the first extraction. The ether layer should now be washed by adding to it 15 mL of water, shaking as before and then running off the aqueous washings into the combined aqueous extracts. The resulting aqueous solution (total of approx. 45 mL) should then be labelled "4-chloroaniline, hydrochloride salt" and set aside.
Now add carefully to the separatory funnel 15 mL of 5% sodium bicarbonate solution. Stopper the funnel and shake it carefully, remembering to release the pressure caused by carbon dioxide evolution at frequent intervals through the tap. When shaking is complete, remove the stopper and set the funnel on a clamp or a support ring, to allow the two layers to separate.
When separation is complete, run off the lower aqueous layer into a second clean 125 mL Erlenmeyer flask, and then add a further 15 mL of 5% sodium bicarbonate solution to the remaining ether solution in the separatory funnel and repeat the extraction procedure. The second 15 mL of aqueous solution may then be run off and combined with 15 mL obtained from the first extraction. The ether layer should now be washed by adding to it 15 mL of water, shaking as before and then running off the aqueous washings into the combined aqueous extracts. The resulting aqueous solution (total of approx. 45 mL) should then be labelled "benzoic acid, sodium salt" and set aside.
The ether solution which remains in the separatory funnel may now be run off into a clean 125 mL Erlenmeyer flask. Wash the inside of the funnel with 5 mL of ether. Anhydrous sodium sulfate (approx. 2 g) is added to the flask in order to dry the ether solution by removing any traces of water which remain (pp.125-126). The mixture should be allowed to stand for several minutes, with frequent agitation, before filtering. This solution is then by gravity filtration ( pg.26; use fluted filter paper see Fig. 1.8 pg.27) into a dry 125 mL Erlenmeyer flask, which has been previously weighed.
[Make sure you wash the Na2SO4 with a few mL of ether and wash the filter paper to get all the organic material into the flask.]
The ether is removed then by evaporation using a rotary evaporator (pp. 144-145) to leave 4-dibromobenzene whose weight should be determined, bottled in a labeled vial. See example of a completed label. Be careful not to overheat the 4-dibromobenzene. 4-Dibromobenzene has an appreciable vapour pressure at room temperature (even as the solid). Calculate the % of 4-dibromobenzene present and record in the experiment's Results section in your lab notebook.
The solution of benzoic acid as the sodium salt must now be treated with dilute acid to recover the benzoic acid. To do this, add dilute (6M) hydrochloric acid slowly and carefully to the solution, with constant swirling, until a dense, white precipitate of benzoic acid is apparent and the solution is acidic about pH = 2 when tested with pH paper. During this addition, carbon dioxide is evolved and care is necessary in order to prevent the solution from frothing out of the flask.
When precipitation of the benzoic acid is complete, separate it from the solution by vacuum filtration using a Buchner funnel (pg.28, Fig. 1.9). The precipitate is then washed with a small amount of ice-cold water, dried on a sheet of filter paper and weighed.
[If you obtained little, or no, precipitate of benzoic acid you probably did not extract the mixture with NaHCO3 correctly and had too dilute a solution - leave your acidified solution in an Erlenmeyer with a filter paper cover and place in your drawer until your next lab period.]
The solution of 4-chloroaniline hydrochloride must now be made basic in order to recover the free amine. Add 10% NaOH solution to your Erlenmeyer containing the 4-chloroaniline hydrochloride, a bit at a time and with stirring; cool the flask in cold water if necessary (you should use about 30-35 mL of 10% NaOH). Check the pH with pH-paper in the latter stages of the addition to ensure that the pH of the solution is 11-12. When the precipitation of the 4-chloroaniline is complete, it may be separated from the solution by filtering on a Buchner funnel (pg.28, Fig. 1.9).
The precipitate is then washed with a little cold water, dried on a sheet of filter paper and weighed.
[If you do not have time to acidify or basify your solutions or evaporate your ether layer, these may be stoppered and stored until your next Laboratory period]