Introduction:
James
V. McCullagh
Department of Chemistry and Biochemistry, Manhattan College, Riverdale, NY
"The ability to produce enantiomerically pure compounds is crucial to the fine
chemicals industries (pharmaceuticals, agrochemicals, fragrances etc.). For
instance, the revenues generated in the pharmaceutical and agrochemical industries
by the production of enantiomerically pure products was estimated at $8 billon
in 2003. The ability to produce enantiomerically pure compounds is probably
most important in the pharmaceutical industry, where approximately 80% of the
drugs currently under development are chiral (2). Enantiomerically pure compounds
can be produced by one of two methods. The first method is by a process referred
to as resolution. This a process by which the two enantiomers of a racemic
mixture are separated. The second method is by asymmetric synthesis. In an
asymmetric synthesis, only one of the two possible enantiomers of the desired
compound is produced. Asymmetric synthesis involves the use of chiral catalysts
such as chiral transition metal complexes or enzymes in key steps of the synthesis.
The use of asymmetric synthesis has increased dramatically over the last few
decades, but resolutions are still the most heavily used method in the large-scale
production of enantiomerically pure compounds industrially (2).

In this experiment, you will analyze the resolution of the medicinally active (S)-(+)- ibuprofen enantiomer from a racemic mixture. Ibuprofen belongs to a class of pharmaceuticals referred to as non-steroidal anti-inflammatory drugs or NSAIDS. It is the active ingredient of Motrin®, Advil® and Nuprin® along with their generic equivalents. Ibuprofen exhibits analgesic, antipyretic and anti-inflammatory properties. This makes it well suited for alleviating minor to moderate aches and pains and in fever reduction. Additionally it has found use in treatment of rheumatic fever, rheumatoid arthritis and osteoarthritis (3). Ibuprofen functions by strongly inhibiting the conversion of arachidonic acid into prostaglandin E2, which serves as a chemical signal for vasodilatation (inflammation) and pain perception. It has been shown for the aryl-substituted proprionic acid NSAIDS, compounds such as ibuprofen, naproxen and related compounds, that of the two enantiomers only one has the desired biological activity. For ibuprofen, the (S)-(+)-enantiomer has the desired pharmacological activity while the (R)-(-) –enantiomer is inactive. With ibuprofen the (R)-(-) –enantiomer has been shown to be non-harmful so currently most commercial ibuprofen is sold in a racemic form (2,3). However, recent research suggests that optically pure (S)-(+)-enantiomer of ibuprofen is more effective and therefore several pharmaceutical companies are developing methods to produce the enantiomerically pure form of ibuprofen and its derivatives (2,4,5). [NOTE: There is value in the R- isomer. It has been found that humans have an enzyme which isomerizes the Inactive R- form to the active S- isomer in vivo.]
|
There are several different types of enantiomeric resolutions. The first type
of resolution discovered was the direct crystallization method. In this method,
the compound
in question, forms half the crystals containing predominantly the R enantiomer
and the other half containing predominantly the S enantiomer (2). The different
crystals, if carefully examined can be physically separated by differences
in their appearances. Alternately, if you have an enantiomerically pure crystal
this can be used as a seed crystal to precipitate the crystals of one enantiomer
preferentially. This process was first used by Louis Pasteur in his groundbreaking
resolution of tartaric acid. This first resolution greatly aided the development
of our understanding of the stereochemistry of organic compounds and led to
the
discovery that many organic molecules have isomers that are non-superimposable
mirror images (2,6). This type of resolution, however, only works with 5 to
10 percent of organic compounds. The remaining organic compounds tend to form
racemic
crystals containing equal amounts of both enantiomers, thereby precluding resolution
using the direct crystallization method.
The other types of resolutions belong to a class of resolutions referred to
as chemical resolutions. These work by temporarily converting the two enantiomers
to be separated into diastereomers. In these cases the racemic mixture to be
separated is reacted with a single enantiomer of second chiral compound (a
resolving
agent). The resolving agent is most often a single enantiomer of another chiral
organic molecule but in some types of resolutions it can be a biological agent,
such as an enzyme, or a transition metal catalyst. By reacting the two enantiomers
of the racemic mixture with the resolving agent, two diastereomeric compounds
will be formed. Unlike enantiomers, diastereomers do not have identical physical
properties, i.e. melting points, boiling points, solubility, chemical relativities
etc… If these differences in physical properties are large enough they
can be used to separate the two diastereomers. After separation the two diastereomers
are converted back to the two enantiomers by removal of the resolving agent.
See Figure 1 for a general overview of the process.

A good resolving agent has several requirements. First, it
must be chiral and available as a single enantiomer (not a racemic mixture).
Second, it must react with the compound to be resolved to form diastereomers
with significant differences in their physical properties. Third, the reaction
that formed the diastereomers must be reversible so that the compound being
resolved can be recovered at the end of the experiment. (Once the diastereomers
are physically separated from each other, the resolving agent must be removed
to yield the individual enantiomers.)"
References
(1) Palleros, D. R., Experimental Organic Chemistry, John Wiley & Sons.
Inc. New York, NY, 2000, p 242-249, 642.
(2) Ager, D., Ed. Handbook of Chiral Chemicals, 2nd ed.; CRC Taylor & Francis
Group: Boca Raton, FL, 2006, p 75-78, 81-82, 97-98.
(3) Delgado, J. N.; Remers, W. A. Wilson and Gisvold’s Textbook of
Organic Medicinal and Pharmaceutical Chemistry, 9th ed.; J. B. Lippincott
Co. : Philadelphia, PA, 1991, p 656 -664
(4) Manimaran, T.; Impastato, F. J.; U.S. Pat. 5,015,764, 1991.
(5) Manimaran, T.; Stahly, G. P.; U.S. Pat. 5,248,814, 1993.
(6) Kauffman, G. B.; Myers, R. D.; J. Chem. Educ. 1975, 52, 777-781.
Work with your partner to analyze the experimental results outlined in the following scheme. One partner will analyze ibuprofen A and the other ibuprofen B. Answer the related questions on the Lab form.
I) Formation and Separation of Diastereomeric Salts
3.06 g (14.8 mmol) of racemic ibuprofen was added to 30 mL of 0.5M KOH and heated in a water bath to 75 oC - 85 oC. 1.9 mL, d = 0.94 g/mL (14.8 mmol) of S-(-)-α-phenethylamine was added dropwise. The solution was stirred at 75 oC - 85 oC for 1 hour and then cooled to room temperature. The diastereomeric salt (Filtrand) was collected by vacuum filtration and washed with 2-3 mL of ice cold water. [The aqueous filtrate in the filter flask was retained. (Compound B)]
II) Compound A – Recovery of a Single Ibuprofen Enantiomer from Filtrand.
The salt from I) was recrystallized with 30 mL of 2-propanol. 25 mL of 2M H2SO4 was added to the salt and the solution stirred for 5 minutes. The aqueous layer was extracted with 3x15 mL portions of MTBE (methyl-t-butylether) which were combined and extracted with 15 mL of water, and then with 15 mL of a saturated NaCl solution. The MTBE was dried with anhydrous sodium sulfate, and the MTBE evaporated producing 1.25 g of ibuprofen A, m.p. 76-78 oC.
II.A) Polarimetry (Lab Text/Guide pp.54-57)
0.99g of Product A was dissolved in 5.0 mL of ethanol, and the optical rotation measured using a sodium lamp and a 10.0 cm cell. The results are provided in Lab form (Part III).
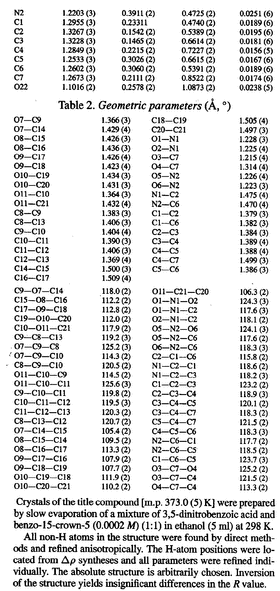
Four researchers collaborated: A. A. Freer, J. M. Bunyan, N. Shankland, and D. B. Sheen. They recrystallized 0.26g of Product A using the solvent system listed at the bottom of the following Table. A crystal was then analyzed by using X-ray diffraction, which produced the coordinates listed in the Table. To view the output graphically, click on the Table.
![]()
III) Compound B– Recovery of a Single Ibuprofen Enantiomer from Filtrate.
25 mL of 2M H2SO4 was added to the filtrate from I) and the solution stirred for 5 minutes. The aqueous layer was extracted with 3x15 mL portions of MTBE (methyl-t-butylether) which were combined and extracted with 15 mL of water, and then with 15 mL of a saturated NaCl solution. The MTBE was dried with anhydrous sodium sulfate, and the MTBE evaporated producing 0.95 g of ibuprofen (oily semi-solid).
III.A) Polarimetry (Lab Text/Guide pp.54-57)
0.76 g of Product B was dissolved in 5.0 mL of ethanol, and the optical rotation measured using a sodium lamp and a 10.0 cm cell. The results are provided in Lab form (Part III).