|
Species
|
Lewis Structure
|
Valence Electrons "Owned" by Central Atom
|
Valence Electron Count of Neutral Central Atom
|
Formal Charge of Central Atom
|
Reactivity
|
|
neutral
|

|
4
|
4
|
0
|
very low
|
|
carbocation
|
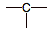
|
3
|
4
|
+1
|
high: strong electron pair acceptor
(ACID)
|
|
free radical
|
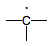
|
4
|
4
|
0
|
high: (due to unpaired electron)
|
|
anion
|
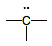
|
5
|
4
|
-1
|
high: strong electron pair donor
(BASE)
|
|
ammonia
|
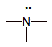
|
5
|
5
|
0
|
weak electron pair donor; weak electron pair acceptor
(BASE)
|
|
ammonium ion
(cation)
|
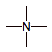
|
4
|
5
|
+1
|
strong electron pair acceptor
(ACID)
|
|
amide ion
(anion)
|

|
6
|
5
|
-1
|
high: strong electron pair donor
(BASE)
|
|
Water
(neutral)
|
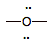
|
6
|
6
|
0
|
weak electron pair donor; weak electron pair acceptor
(ACID or BASE)
|
|
hydronium ion
(cation)
|
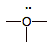
|
5
|
6
|
+1
|
strong electron pair acceptor
(ACID)
|
|
hydroxide ion
(anion)
|
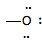
|
7
|
6
|
-1
|
strong electron pair donor
(BASE)
|
|
bonded hydrogen
(neutral)
|
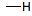
|
1
|
1
|
0
|
low unless
activated by neighboring
atom(s)
|
|
hydride
(anion)
|
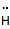
|
2
|
1
|
-1
|
strong electron pair donor
(BASE)
|
|
proton
(cation)
|
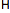
|
0
|
1
|
+1
|
strong electron pair acceptor
(ACID)
|
|
hydrogen
atom (free radical)
|
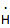
|
1
|
1
|
0
|
high: (due to unpaired electron)
|