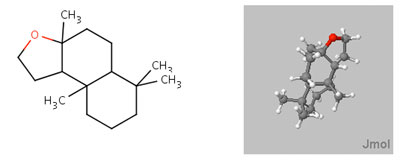
 Ambrox |
|
Define the distances (Å) in the spatial triangle for a synthetic compound, Analog A, in the jmol model below the ambrox images. Determine if it will smell like ambergris or not based on the Triaxial Rule. Be sure to clearly illustrate the triangle and appropriate interatomic distances to support your conclusion.
[Note: The ring system of the Analog is slightly different than ambrox, but the Triaxial Rule still applies.]After completing your analysis for Analog A, if you have not already done so repeat the process for Analog B.
(I) 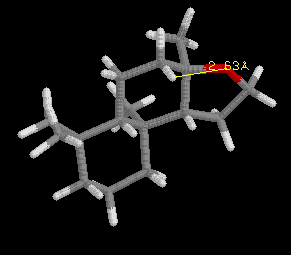 |
(II) 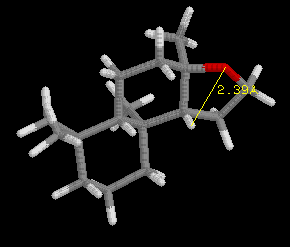 |
(III) 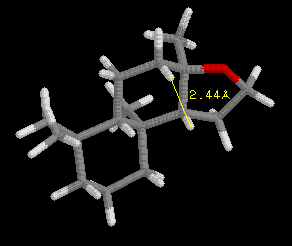 |
Analog A: