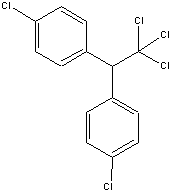

DDT and DDE are displayed above. Save .pdb files of DDT and DDE to your desktop.
Using RasMol, which can be downloaded at: http://mc2.cchem.berkeley.edu/Rasmol/.
Click on the RasMol bond distance feature to determine the C-C interatomic distance
between the non-ring carbons in DDT and DDE. Record those values and explain
why one is shorter than the other. Provide a mechanism for the conversion of
DDT to DDE.
|