EXPLORATION 3:
What feature makes a carboxyl group reactive toward nucleophiles,
and how can we extend the principle to cover central atoms besides carbon?
Why is this an important question?
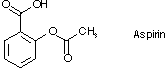
We have already seen some indications of how reactive carbonyl groups are toward nucleophiles. Early in the course, we saw how easy it was to convert aldehydes and ketones into alcohols by addition of hydride (from lithium aluminum hydride or sodium borohydride) or alkyl groups (from organolithium or organomagnesium halides). Carboxyl groups carry the same reactive carbonyl group as do aldehydes and ketones, but with an important difference: the carbonyl group is bonded to an electronegative element such as halogen, oxygen or nitrogen. Aspirin is a good example of a carboxyl (or carboxylic acid) derivative, since it contains both an ester group and a carboxylic acid group.

There are a number of important classes of carboxylic acid derivatives, including acid chlorides (acetyl chloride, for example), acid anhydrides (acetic anhydride, for example), esters (methyl acetate, for example) and amides (acetamide, for example):

Different derivatives have different reactivities. A convenient comparison substance for reactivity would be water, which which carboxylic acid derivatives can undergo a hydrolysis reaction. Acetyl chloride reacts violently with water; acetic anhydride reacts exothermically, but over minutes or hours; methyl acetate reacts only over the course of several days or weeks; and acetamide may take months to react completely. In each case the products are acetic acid, plus the conjugate acid of the group that is displaced. The substitution mechanism, based on many studies spanning the past fifty years or more, is one that we can call "addition-elimination". In this mechanism, water adds to the carbonyl group, then a leaving group is expelled. Proton transfer can occur either before, during or after the leaving group leaves.
As we will see, this fundamental reaction mechanism applies widely in the formation (synthesis) and breakdown not only of carboxylic acid derivatives, but also in the chemistry of many organophosphorus and organosulfur compounds as well.Worksheet and group discussion
Pick a group spokesperson and a group secretary. Your task is to develop a table. In the first column list the structures of : Acetyl chloride, phosgene, thiophosgene, thionyl chloride, sulfuryl chloride, phosphorus oxychloride, phosphorus sulfochloride. In a second column draw the structure of the product from the reaction of each compound in the first column with water. In a third column draw the structure of the product from the reaction of each compound in the first column with ammonia.
Next, predict the relative reactivities of the following compounds with water.
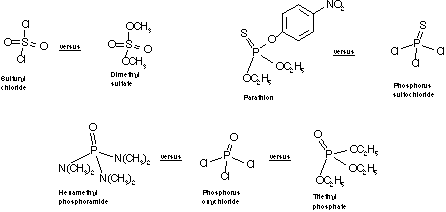
If you are uncertain, consider the relative reactivities of acetic acid derivatives. Remind yourself that since the attacking reagent is water in each case, and the element to which it adds is the same, you must consider differencesand look for a pattern. Focus on the group that leaves (is displaced) in the hydrolysis. What might make a group leave more or less easily? When time is up, your spokespeople will be asked to present either your predictions about relative reactivity, and/or your predicted reaction products.
EXPLORATION 3B:
How can the addition-elimination mechanism be applied to the synthesis of organosulfite, organosulfate, or organophosphate compounds?
Creating the context
Before we go on to more complex examples, we ought to make sure that we're comfortable with some fundamental examples of conversions via the addition-elimination mode of substitution. In general, we start with a more reactive compound (such as a reactive inorganic precursor) , and go toward progressively less reactive products, as a way of maintaining selectivity along the way. Where different groups are to be added to the same central atom, amounts of reactants must be carefully metered at each step.Worksheet and Group Discussion
Pick a new spokesperson and a new secretary. Your task will be to propose a step-by-step mechanism for one of the following conversions (your conversion will be on the slip of paper you are about to receive):
A
B
C
D
E
F
G
When you are done, please put the completed mechanistic scheme on the board for class discussion.
EXPLORATION 3C:
You will be given a list of 6 reactants. Three from list A) and three from list B) Provide the nine products for the reactions of the combinations in a three x three matrixStarting materials:
A) Components with a reactive center: (acetyl chloride, phosgene, thiophosgene, sulfuryl chloride, chlorosulfonic acid, benzenesulfonyl chloride, phosphorus oxychloride, phosphorus sulfochloride);B) Components that can add to the reactive C, S, or P center: (water, methanol, ethanol, 2-propanol, methanethiol, 2-mercaptodiethylsuccinate, phenol, 4-nitrophenol, ammonia, methylamine, dimethyl amine and aniline.)
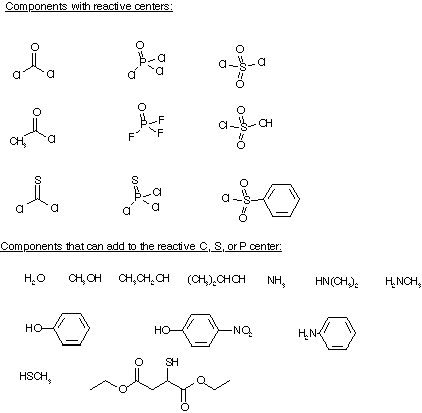
The matrix is to be completed and turned in.