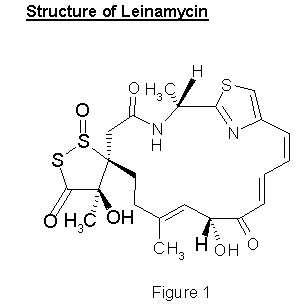
LEINAMYCIN
LEINAMYCIN; CAS REGISTRY #: 120500-15-4
IUPAC SYSTEMATIC NAME:
MOLECULAR FORMULA: C22H25N2O6S3

MOLECULAR WEIGHT: 509.65 g/mol
MELTING POINT: 155 oC
DENSITY: 1.34 g/ml
SPECIFIC ROTATION (MeOH): [a ]25D -140 °
TLC (solvent 95% MeOH): Rf = 0.100
UV MAX (MeOH): 211 nm (14,000), 322 nm (12,000)
1H NMR (400 MHz, DMSO):
13C NMR (100 Mhz, DMSO):
MASS SPECTROSCOPY (HRFAB):
NARRATIVE:
Leinamycin is a recently discovered antitumor antibiotic and was first isolated from a culture broth of Streptomyces sp. in 1989 by Dr. Y. Kanda and Dr. T. Fukuyama at Kyowa Hakko Kogyo Co., Ltd. in Tokyo, Japan. In the course of continued screening for new antitumor antibiotics, the chemists at Kyowa Hakko have found that four additional actinomycetes isolated from soil samples collected in various areas of Japan are also producers of Leinamycin. Based on morphology and whole cell analysis, all Leinamycin producing strains were identified as of the species Streptomyces.
During the last three decades a very wide range of oxazole and thiazole containing secondary metabolites such as Leinamycin have been isolated and characterized from Nature. Furthermore, several of these metabolites have been shown to exhibit profoundly useful biological properties and antitumor activities.
The Streptomyces metabolite Leinamycin contains an unusual 1,3-dioxo-1,2-dithiolane moiety, which is connected to the 18-membered lactam ring through a spiro linkage, and can be classified as a new group distinct from any known classes of antibiotics or microbial metabolites. The structure of Leinamycin (see Figure 1) was revealed by X-ray crystallography and the absolute configuration of the antibiotic was determined by spectroscopic and chemical analysis.
Studies performed at Kyowa Hakko in 1990 have shown that Leinamycin exhibits potent antitumor activity against murine experimental tumors leukemia P388 and sarcoma 180. The compound was also shown to be active against Gram-positive and Gram-negative bacteria. Leinamycin as do other similar antitumor agents such as Mitomycin, Bleomycin, and Adriamycin is thought to interfere with DNA through cross-linkage, strand breakage, intercalation, or other kinds of interactions, which result in inhibition of nucleic acid synthesis. Thus, the primary cellular target of Leinamycin appears to be DNA. It binds DNA and causes single-strand break at low concentrations, which may account for the potent antitumor activity.
Leinamycin is not thought to be a cure for leukemia P388 or sarcoma 180, but it does inhibit the tumorÕs growth and progress. Leinamycin has not yet been used extensively as a treatment for any of these cancers in humans as detailed studies on antitumor spectra and toxicity are still in progress and a safe level for humans is still yet to be accurately determined. Futhermore, side effects will also be taken into consideration as like in other similar anticancer agents, Leinamycin may cause hair loss, dizziness, and low blood counts.
When Leinamycin was first isolated in 1989, a culture broth of 2,000 liters fermenter, 1,000 liters of fermentation beer, 430 liters of methanol and other solvents were needed to produce only 10 g of pure crystals of Leinamycin. Today synthesizing Leinamycin in the literature today shows that 4 synthetic schemes consisting of over 20 synthetic steps will produce a fair amount and a significant 82% yield but still has yet to be cost effective as there is a fairly large amount of solvents and materials still used. Hopefully in the near future, a total synthesis with a fewer number of synthetic steps will be determined and will therefore improve the economic efficiency in the manufacture of the product.
SYNTHESIS:
The total synthesis of Leinamycin with its unique structural features as well as interesting antitumor activities prompted the organic chemists of the Tokyo Research Laboratories and Kyowa Hakko in Japan, and the Department of Chemistry at Rice University in Houston, Texas to undertake the first total synthesis of Leinamycin.
The relative configuration of Leinamycin was determined by an X-ray crystallographic analysis, and the absolute stereochemistry was deduced and the desired isomer (+) was the critical intermediate for the construction of the characteristic spiro dithiolanone moiety, which was reported to be essential for the biological activities.
In conclusion, the following pages outline the total synthesis of Leinamycin proposed by Y. Kanda and T. Fukuyama and their colleagues at Tokyo Research Laboratories and Kyowa Hakko in Japan, and the Department of Chemistry in Rice Univerity published in the May 17, 1993 issue of the Journal of the American Chemical Society.
Scheme I: 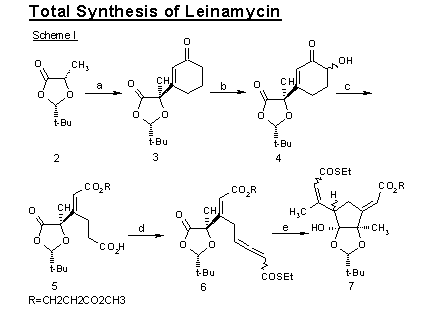
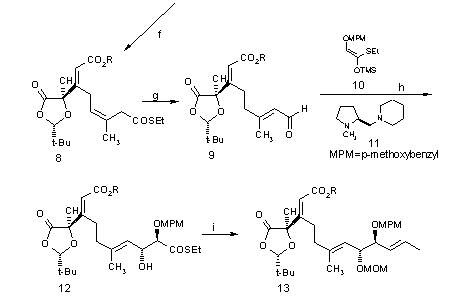
Reagents and Conditions: (a) LDA, THF, 3-ethoxy-2-cyclohexen-1-one, -78 °C, 3 M HCl, 23 °C, 1 h (69%); (b) TMSOTf, Et3N, Et2O, 0 °C, 10 min.; m-CPBA, NaHCO3, CH2Cl2/H2O, 0 °C, 20 min.; 3 M Hcl, THF 23 °C, 15 min. (91%, 3 steps); (c) H5IO6, THF, 23 °C, 2 h, then HO(CH2)2CO2Me, DMAP, 23 °C, 1 h (59%, 2 steps); Jones reagent, acetone, 0 °C, 10 min. (99%); (d) (COCl)2, CH2Cl2, 23 °C, 1 h; Ph3P==CHCOSEt, Et3N, CH2Cl2, 23 °C, 20 min. (73%, 2 steps); (e) Me2CuLi, Et2O, -78 °C; (f) Me2CuLi (4 equiv.), PhOH (6 equiv.), Et2O, -78 °C, 20 min. (87%); (g) Et3SiH, 10% Pd/C, acetone, 23 °C,1 h; DABCO, CH2Cl2, 23 °C, 2 h (92%, 2 steps); (h) Sn(OTf)2, 11, n-Bu2Sn(OAc)2, CH2Cl2, 23 °C, 30 min., then 10,9, -78 °C, 18 h (92%); (I) MEOCH2Cl, i-Pr2NEt, CH2Cl2, reflux, 5 h (91%); Et3SiH, 10% Pd/C, acetone, 23 °C, 1 h (99%); CHI3, CrCl2, THF, 23 °C, 40 min. (66%).
Scheme II: 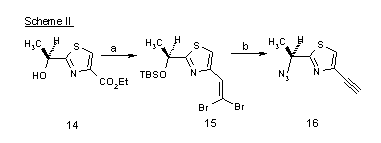
Reagents and Conditions: (a) TBSCl, imidazole, DMF, 23 °C, 24 h; LiAlH4, THF, 0 °C, 10 min.; DMSO, (COCl)2, CH2Cl2, -78 °C, 15 min., then Et3N, -78 Æ 23 °C, 15 min.; CBr4, PPh3, CH2Cl2, 0 °C, 10 min. (86%, 4 steps); (b) n-BuLi, THF, -78 °C, 20 min.; n-Bu4NF, THF, 0 °C, 2 h (86%, 2 steps); HN3, PPh3, DEAD, toluene, 0 °C, 20 min. (99%).
Scheme III: 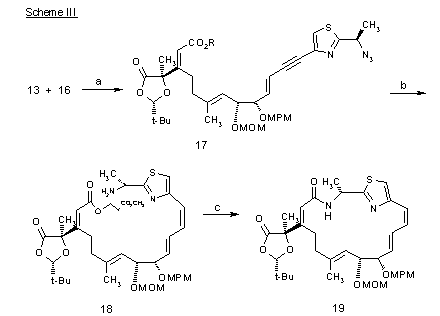

Reagents and Conditions: (a) PdCl2 (Pph3)2, CuBr, Et3N, THF, 23 °C, 30 min. (88%); (b) Zn, AcOH, EtOH, 23 °C, 30 min. (99%); H2, Lindlar catalyst, quinoline, MeOH, 23 °C, 2 h (74%); (c) DBU, CH3CN, 23 °C, 1.5 h (99%); BOP-Cl, i-Pr2NEt, toluene, 60 °C, 20 min. (91%).
Scheme IV: 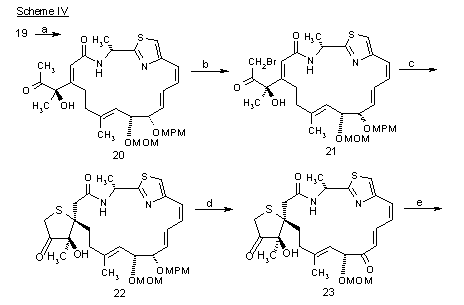
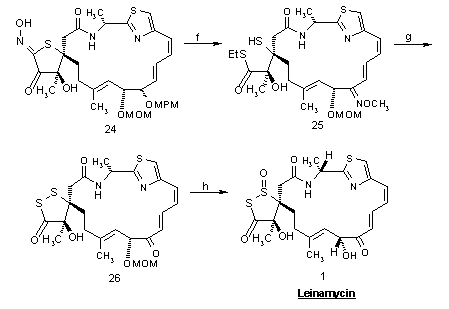
Reagents and Conditions: (a) p-TolSO2Me, n-BuLi, -78 Æ 0 °C, 30 min. (94%); Al(Hg), THF/H2O, 23 °C, 40 min. (95%); (b) TMSCl, DBU, CH2Cl2, reflux, 11 h; NBS, CH3CN/ H2O, 0 °C, 5 min.; 10% HClO4, THF, 23 °C, 8 h (77%, 3 steps); (c) H2S, Et3N, THF, 23 °C, 2 h (80%); (d) DDQ, CH2Cl2/ H2O, 23 °C, 45 min. (95%); (e) i-AmONO, NaOMe, 23 °C, 1.5 h (74%); (f) MeONH2 · HCl, pyridine, MeOH, 23 °C, 30 min. (94%); 2,6-dimethylbenzoyl chloride, pyridine, CH2Cl2, 23 °C, 10 min.; EtSH, KH, THF, 23 °C, 30 min. (52%, 2 steps); (g) NaSH, THF, 23 °C, 20 min., then I2, 23 °C (82%); 35% HCHO, 3 N HCl, acetone, 23 °C, 77 h (64%); (h) 3 N HCl, AcOH, 0 °C, 45 min. (61%); m-CPBA, THF, 0 °C, 45 min. (84%).
Four Reactions Generically Identified in SolomonsÕ Text:
1) Reaction (a) of Scheme I - LDA used in a directed aldol reaction., p.788, H.3.
2) Reaction (e) of Scheme I - Ketones from Lithium Dialkylcuprates., p.714, 16.5C.
3) Reaction (a) of Scheme II - Electrophilic Substitution Reactions of Aromatic Heterocyclic Amines, p.953, J.3.
4) Reaction (b) of Scheme III - Syn Addition of Hydrogen: Synthesis of Cis- Alkenes, p.293, 7.7A.
REFERENCES:
Kanda, Yukata & Fukuyama, Tohru, et al. ÒTotal Synthesis of Leinamycin.Ó Journal of the American Chemical Society, 1993, 115, 8451-8452.
Asai, Akira, et al. ÒThiol-Medicated DNA Alkylation by the Novel Antitumor Antibiotic Leinamycin.Ó Journal of the American Chemical Society, 1996, 118, 6802-6803.
Hirayama, Noriaki & Matsuzawa, Etsuyo S., et al. ÒMolecular Structure of a Novel Antitumor Antibiotic Leinamycin.Ó Chemistry Letters, 1993, 11, 1957-1958.
Pattenden, Gerald, et al. ÒSynthetic Studies with Natural Oxazoles and Thiazoles.Ó Journal of Heterocyclic Chemistry, 1992, 29 (3), 607-618.
Yamada, Haruo, et al. ÒStereoselective Intramolecular Micheal Reaction of the 18-Membered alpha, beta-Unsaturated Macrolactam: MM2 Transition Structure Models.Ó Heterocycles, 1996, 43 (2), 267-270.
Hara, Mitsunobu, et al. ÒDNA Strand Scission by the Novel Antitumor Antibiotic Leinamycin.Ó Biochemistry, 1990, 29, 5676-5681.
Hara, Mitsunobu & Takahashi, Isami et al. ÒDC 107, a Novel Antitumor Antibiotic Produced by a Streptomyces sp.Ó The Journal of Antibiotics, 1989, 42, 333-335.
Hara, Mitsunobu & Asano, Kozo et al. ÒLeinamycin, a New Antitumor Antibiotic from Streptomyces; Producing Organism, Fermentation and Isolation.Ó The Journal of Antibiotics, 1989, 42, 1768-1773.
Kanda, Yukata & Takahashi, Isami. Internet and email communication for physical and physicochemical data information. Email addresses: ÒYukata KandaÓ <ykanda@kyowa.co.jp>, ÒIsami TakahashiÓ <itakahashi@kyowa.co.jp>