Applications of Molecular Modeling
Conformational & Structural Exercises based on Computational
Chemistry
There are many definitions for a model. Two of them from
Webster's Dictionary are most appropriate to applications in organic chemistry.
The first is: "a description or analogy used to help visualize something
that cannot be observed". The second is: "a system of postulates, data and
inferences presented as a mathematical description of an entity". Molecular
Modeling incorporates both definitions.
The background materials in the following links give an idea of the mathematics
involved and the basis for computationally generated,
energetically minimized
images. There are many public domain and commercial software programs that
can be used to do this. You have already applied one of these approaches
in the WebMO/ Dipole Moment exercise that you completed earlier. The structures
in this exercise have been generated and energetically minimized for your
use with MacSpartan and stored as .pdb files. You are most welcome and encouraged
to continue to use WebMO. You may use WebMO to generate the structures
in the exercise and compare the values to those from MacSpartan.
Introduction:
Molecular Orbital Theory / Computational Chemistry / Molecular
Mechanics:
The Schrodinger equation is the basis for quantum mechanics
and provides a mathematical model for atomic orbitals. It demands enormous
computational power to solve for all but the simplest of atoms and focuses
on nuclei, electrons and orbitals. It is virtually impossible to apply
it to organic molecules. Several alternatives have evolved which provide
approximations through simplification of the classical Schrodinger equation.
These alternatives are computational techniques or methods referred to
as "Molecular Mechanics" and describe atoms as classical particles whose
interactions are represented by potential energy functions based on mechanical
force field(s). The simplest model is the sum of all of the energy terms
for the structure. An improvement considers various possible conformations
for the molecule, and total energy is calculated by considering all of
the molecule's possible conformations, summing the energies from individual
bond characteristics such as bending, stretching and bond angle, torsional
strain and weak repulsive forces. These calculations produce minimized
energy structures which are energetically favored conformations for the
molecule. A further refinement is Molecular Dynamics which involves additional
calculations that include the particle's acceleration and velocity and
provide a better correlation to observed data for molecules' physical properties.
These approaches offer great possibilities in developing strong visual
models for many different large and small molecules. They are widely applied
in molecular biology and in bioorganic chemistry particularly for imaging
proteins, RNA, DNA as well as small organic molecules.
Molecular orbital theory has produced a wide range of computational
methods that perform molecular calculations:
|
Numerical Methods
|
|
Integral Method
|
|
Ab Initio Methods
|
|
Semi-Empirical MO-SCF Methods
|
|
Approximate MO Methods
|
There are also many, many variations within each. See the Computational
Chemistry List (CCL) for a list of
the hundreds of computational programs that are available.
Conformational & Structural Exercises
There are two principal bond features that relate to conformation:
(1) There is free rotation about single bonds (sigma bonds), but due to
repulsion, spatial distances between atoms are minimized. Therefore, different
forms of rotational possibilities will have higher or lower frequency of
occurence depending on the proximity and interaction of atoms. (2) Carbon-carbon
double bonds have fixed geometry.
Key glossary terms that you are to become familiar with:
rotamer, conformer,
syn,
gauche, eclipsed, anti, staggered, chair, boat, twist boat, axial, equatorial,
cis (Z), trans (E), dihedral angle, inter-atomic distance, torsional strain
See:
1) Dublin
City University's: Introduction to Conformational Analysis
2) Western
Washington University Virtual Molecular Model Kit
3) University
of Illinois, Chicago: Confromational Analysis
Conformational Analysis
Part I:
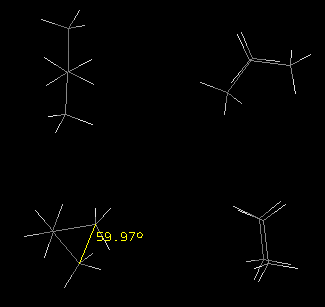
A) Acyclic conformers: (Butane)
Refer to the following gauche, anti, eclipsed and staggered images of
butane and complete the instructions which follow.
The approximate dihedral angle for conformer number one was determined
with a RasMol feature, which is also available with jmol. The angle is relative
to the C1-C4 carbons
and is indicated in yellow. View the four conformational files of butane
that are linked below.
- Draw Newman projection formulas for the four butane conformers:
- Using a viewing perspective through the C2-C3 bond,
identify each by name.
- Draw a relative, torsional energy diagram starting with a 0o dihedral
angle and rotate about the C2-C3 bond through 360o
labeling the conformations of all of the peaks and valleys. Circle
the least favored conformer.
B) Conformers in cyclic systems: (Cyclohexane / Ambrox)
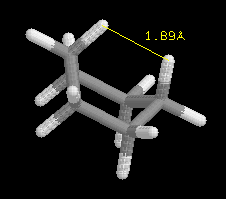
Cyclohexane can exist in a boat or chair conformation. The boat is illustrated
below with the interatomic distance in yellow for the "Bowsprit Flagpole"
hydrogens to 3 significant figures. Click on the image and change the view
of the structure by selecting Render → Scheme → CPK
Spacefill → Atoms → 75%
vanderWaal's. The image
gives
an indication of the significant interaction between these two atoms. Their
surfaces overlap, which is observed at 100% vanderWaal's. Generally, if
the hydrogen atoms are closer than 2.40 Angstroms
they will interact "sterically" which is an energetically unfavorable interaction.
Steric effects are very important in explaining the conformation and
chemical behavior of organic molecules. What is the jmol interatomic distance
betweeen the "Bowsprit Flagpole" hydrogens to four significant figures? Is
the difference between 3- and 4- "significant"? Briefly
explain.
1) Draw line structures for each of the following di-substituted
cyclohexane chair forms and label the substitutents
as being axial (a) or
equatorial. Then draw the chair flipped and
label the substituents as being axial (a) or equatorial.
2) Identify each molecule
of the following four substituted cyclohexane models as cis-
or trans-, and if it is the most energetically stable of the two possible
chair forms and why.
Conformational Analysis
Part II:
Ambergris: Ambrox & Ambreine / Synthetic
Homologs
Herman Melville describes ambergris in the epoch novel, Moby
Dick:
".......ambergris is soft, waxy, and so highly fragrant and
spicy, that it is largely used in perfumery, in pastiles, precious candles,
hair-powders, and pomatum. The Turks use it in cooking, and also carry
it to Mecca, for the same purpose that frankincense is carried to St. Peter's
in Rome. Some wine merchants drop a few grains into claret, to flavor it.
Who would think, then, that such fine ladies and gentlemen should regale
themselves with an essence found in the inglorious bowels of a sick whale!
Yet so it is."
Moby Dick or The Whale was written by Melville
in 1851. If you are interested in reading the novel, it is available on
the Web: http://www.americanliterature.com/MD/MDINDEX.HTML
For further background information on ambergris, ambrox
and ambreine see Dr. Randy Ralph's comprehensive
site: "Ambergris: A Pathfinder and Annotated Bibliography" http://www.netstrider.com/documents/ambergris
Since the decline of whaling and the restriction of global whaling operations,
ambergris has not been commercially used for some time. With the loss of
this material, the perfume industry turned to organic synthesis to prepare
a substitute, and many, many compounds were synthesized and tested. One
that was selected for use is Ambrox. In analyzing the correlation between
structure and the smell of the synthesized analogs it was found that certain
positions and interatomic distances were critical for the bio-activity.
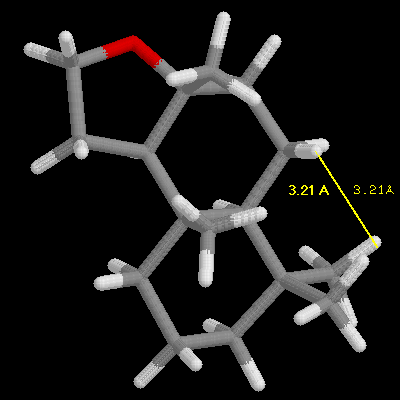
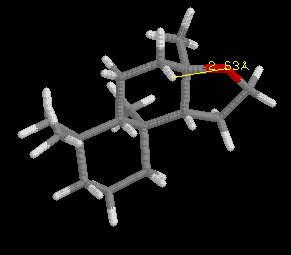
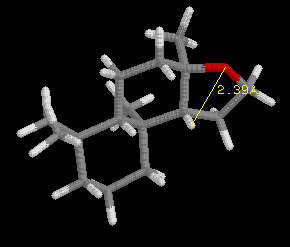
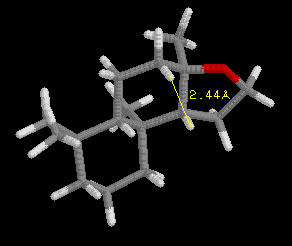
This discovery was framed as the "ambergris triangle" which is illustrated
by the examples in the following three images, and later became know as
the "Triaxial Rule". In order to smell like ambergris the analogs must
have two axial hydrogens within 2.38 Angstroms +/-0.35 A of each other,
and one hydrogen must be within 2.90 Angstroms +/-0.40 A of an oxygen atom
in an adjacent ring, with the other hydrogen 2.45 Angstroms +/-0.35 A from
the oxygen atom. Ambrox meets these criteria as illustrated in the examples
with RasMol.
Define the distances in the spatial triangle for Analog
A and Analog B, and determine
if one, or the other, or both will smell like ambergris. Be sure to clearly
state your conclusion. Note: the ring system is different than Ambrox,
but the Rule still applies.
© Copyright 1997-2008 R.J. Rusay