Crystal Growth / Aqueous Solutions
|
There are many types of different substances that
will produce crystals grown from an aqueous solution: sugar and salt for
example. Crystals begin to grow when the amount of the substance that
is dissolved (the solute) exceeds the amount that in this case
the water (the solvent) is capable of dissolving. The maximum amount
of solute dissolved in the water produces a solution that is saturated.
If additional solute is added, it will not dissolve. By raising the temperature
of the water it is possible to dissolve more solute such as sugar in hot
coffee than would be possible at lower temperatures. Each substance will
have a characteristic solubility that can be experimentally determined.
Typical representation of this data is graphical and referred to as a
solubility curve.
Growing a single crystal such as the NIF-KDP crystal is a very demanding
and difficult challenge as the lab
photos show. There is a strong tendency for crystals to spontaneously
nucleate, i.e., to have many small crystals growing at the same time rather
than having a single one grow to a very large size. To be successful in
growing a single crystal, the correct regimen must be determined and maintained.
Consider the following observations, graphs, diagrams and data. Can you
identify the key variables and select appropriate growth conditions to
grow a single crystal that has the dimensions of the crystal in the image
at the top of the introduction
page and in the time that it took to grow the crystal?
|
|
Crystal growth from an aqueous solution is based
on the existence of metastable regions where spontaneous formation of
crystalline nuclei in the solution is impossible, but in this region,
it is possible to grow a single crystal from a seed.
|
|
|
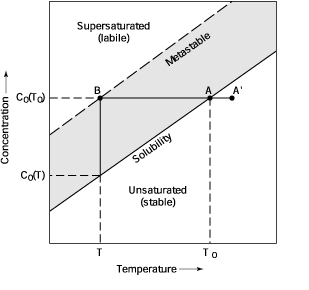
Fig. 4 Phase diagram for a binary
solid-liquid system
|
Figure 4 presents a part of a classical
phase diagram of a two-component system for a solute with a positive temperature
coefficient of solubility dCo/ dT > 0 . The
diagram can be described in terms of three zones: (1) the stable zone
of unsaturated solution where crystal growth is impossible; (2) the metastable
zone where spontaneous crystallization is improbable but crystal growth
on a seed occurs, and (3) the labile zone of spontaneous nucleation.
Cooling the solution with concentration C from the initial point A´
leads to the point A on the solubility curve. At this point, the solution
becomes saturated and can exist in equilibrium with the solid if C =Co(To).
Further cooling leads to a state where the concentration of the solute
C is higher that the equilibrium concentration Co(T) at a given
temperature, and the solution becomes supersaturated. However, spontaneous
formation of the solid phase does not occur in this region until a point
B on the metastable boundary is reached. If crystallization in a solution
saturated at the temperature To starts at the temperature T,
the position of the metastable boundary can be expressed in the units
of maximum overcooling D T max= To - T, the maximum absolute
supersaturation |
|
D Cmax = Co (To)
- Co (T),
|
| or in units of relative supersaturation:
|
| s max
= D Cmax / Co (T) |
| One more parameter that characterizes the metastable
zone is the induction period ti . It corresponds to the time during which
the system can remain without spontaneous nucleation at constant supersaturation
within the metastable zone. |
|
|
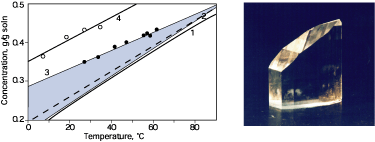
Fig. 5. (a) Metastable zone of DKDP solutions.
(1) Monoclinic phase solubility; (2) Tetragonal phase solubility; (3)
Metastable boundary in the presence of a growing crystal; (4) Metastable
boundary without crystals. The region of tetragonal crystals growth is
shaded. For comparison the data of Ref. [60] are shown (dotted line).
(b) Monoclinic DKDP crystal grown by rapid growth technique at Rz = 16
mm/day. Crystal height is 13 cm.
|
It is possible to grow
a single crystal without spontaneous nucleation only within the metastable
zone. Additionally, the full period of single crystal growth tg should
be less than induction time ti > tg. The practical problem is how to
estimate the width of the metastable zone for a particular crystallization
system. What should this width be in order to provide for successful growth
of a single crystal at high supersaturation during a time that, in the
case of large crystals, can exceed one to two months? Experimentation
using preselected, constant supersaturation concentrations and constant
temperatures provides a guide.
|
|
|
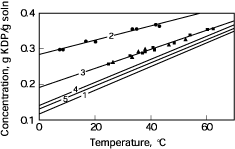
| Fig. 6. Stability of supersaturated
KDP solutions: (1) - solubility curve; (2), (3) - metastable boundaries
of solutions without (*) and with (n) a growing crystal, respectively;
(s) experiments with the empty platform; (4), (5) traditional level of
stability from literature data. |
| Increasing supersaturation
is the simplest way to accelerate crystal growth. However, avoiding spontaneous
nucleation at high supersaturation is a major challenge that must be overcome. |
|
|
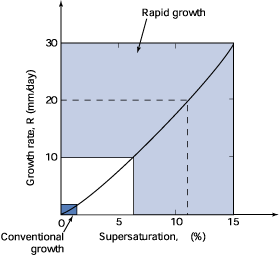
Fig. 7. Approximate comparison
of the regions of conventional and rapid growth of KDP crystals (about
40°C).
|
Growth rates can also be increased
by increasing the temperature. This means that the higher the temperature,
the lower the supersaturation required to obtain the same growth rate.
|
|
|
| Fig. 8. Temperature dependencies s(t) corresponding
to approximately the constant growth rates of KDP crystals Rz: 10, 20,
30, 40, 50, 60 mm/day. The dashed line is the boundary of the metastable
zone. |
Actual
Data Table
|
| Generally, single crystals can be grown by either
a temperature reduction method or using an isothermal method.
The last two parameters to consider are: purity and mass transfer,
the latter of which is directly affected by hydrodynamic conditions
(e.g.. stirring). The growth rate can be increased by utilizing highly
pure raw material, as well as by increasing the velocity of the solution
flow relative to the surface of a growing crystal. The increase in growth
rate due to chemical purity and hydrodynamic conditions, is generally
limited to a factor of two.
|
|
|