Chem 226: Assignments / Activities
Reading/Homework * Worksheets
* Lab Schedule * Experiments
* Lab Techniques & Videos
Reading / Homework:
Key textbook & class topics are below and emphasized
in Bold
Red. Webassign, Worksheet,
and ACS problems will serve as examples for many of the types of
questions
that will be on exams and quizzes.
(In-chapter textbook problems are also important and relate to pre-class
preparation and i-clicker in-class questions. Attempting end of chapter problems
is encouraged as
your time allows.)
 Webassign
Homework .
All assignments are to be done individually. Due dates are embedded on line
and these homework problems should be your main focus along with the collaborative/group
Worksheets.
Webassign
Homework .
All assignments are to be done individually. Due dates are embedded on line
and these homework problems should be your main focus along with the collaborative/group
Worksheets.
ORGANIC CHEMISTRY (Carey 7th ed)
Electronic Structure, Bonding and Shape:VSEPR (Chem
120/121 Review);
Acids and Bases (Chem 120/121 Review).
1.1 Atoms, Electrons, and Orbitals 9
1.2 Ionic Bonds 12
1.3 Covalent Bonds, Lewis Structures, and the Octet Rule 14
1.4 Double Bonds and Triple Bonds 16
1.5 Polar Covalent Bonds and Electronegativity 16
Electrostatic Potential Maps 19
1.6 Structural Formulas of Organic Molecules 19
1.7 Formal Charge 22
1.8 Resonance 24
1.9 The Shapes of Some Simple Molecules 29
Molecular Modeling 30
1.10 Molecular Dipole Moments 32
1.11 Curved Arrows and Chemical Reactions 33
1.12 Acids and Bases: The Arrhenius View 35
1.13 Acids and Bases: The Brønsted–Lowry View 36
1.14 What Happened to pKb? 40
1.15 How Structure Affects Acid Strength 41
1.16 Acid–Base Equilibria 45
1.17 Lewis Acids and Lewis Bases 48
1.18 Summary 49
Introduction to Organic Compounds:
Functional Groups and Representations of Structure.
HYDROCARBONS I: (Chapters 2. & 3. Alkanes)
2.1 Classes of Hydrocarbons 59
2.2 Electron Waves and Chemical Bonds 60
2.3 Bonding in H2 : The Valence Bond Model 61
2.4 Bonding in H2 : The Molecular Orbital Model 63
2.5 Introduction to Alkanes: Methane, Ethane, and Propane 64
Methane and the Biosphere 65
2.6 sp 3 Hybridization and Bonding in Methane 66
2.7 Bonding in Ethane 68
2.8 Isomeric Alkanes: The Butanes 68
2.9 Higher n-Alkanes 68
2.10 The C5H12 Isomers 69
2.11 IUPAC Nomenclature of Unbranched Alkanes 71
What's in a Name: Organic Nomenclature 72
2.12 Applying the IUPAC Rules: The Names of the C6 H14 Isomers 73
2.13 Alkyl Groups 74
2.14 IUPAC Names of Highly Branched Alkanes 76
2.15 Cycloalkane Nomenclature 77
2.16 Sources of Alkanes and Cycloalkanes 78
2.17 Physical Properties of Alkanes and Cycloalkanes 78
2.18 Chemical Properties: Combustion of Alkanes 82
2.19 Oxidation–Reduction in Organic Chemistry 85
Thermochemistry 86
2.20 sp 2 Hybridization and Bonding in Ethylene 89
2.21 sp Hybridization and Bonding in Acetylene 91
2.22 Which Theory of Chemical Bonding Is Best? 92
2.23 Summary 93
Alkanes and Cycloalkanes: Conformations and cis–trans
Stereoisomers 102
3.1 Conformational Analysis of Ethane 104
3.2 Conformational Analysis of Butane 107
Molecular Mechanics Applied to Alkanes and Cycloalkanes 109
3.3 Conformations
of Higher Alkanes 110
3.4 The Shapes of Cycloalkanes: Planar or Nonplanar? 110
3.5 Small Rings: Cyclopropane and Cyclobutane 111
3.6 Cyclopentane 112
3.7 Conformations of Cyclohexane 112
3.8 Axial and Equatorial Bonds in Cyclohexane 113
3.9 Conformational Inversion (Ring Flipping) in Cyclohexane 115
3.10 Conformational Analysis of Monosubstituted Cyclohexanes 116
3.11 Disubstituted Cycloalkanes: cis-trans Stereoisomers 119
Enthalpy, Free Energy, and Equilibrium Constant 120
3.12 Conformational Analysis of Disubstituted Cyclohexanes 121
3.13 Medium and Large Rings 125
3.14 Polycyclic Ring Systems 125
3.15 Heterocyclic Compounds 128
3.16 Summary 129
FUNCTIONAL GROUPS
4. Alcohols & Alkyl Halides
4.1 Functional Groups 139
4.2 IUPAC Nomenclature of Alkyl Halides 141
4.3 IUPAC Nomenclature of Alcohols 142
4.4 Classes of Alcohols and Alkyl Halides 142
4.5 Bonding in Alcohols and Alkyl Halides 143
4.6 Physical Properties of Alcohols and Alkyl Halides: Intermolecular Forces
144
4.7 Preparation of Alkyl Halides from Alcohols and Hydrogen Halides 148
4.8 Mechanism of the Reaction of Alcohols with Hydrogen Halides 149
4.9 Potential Energy Diagrams for Multistep Reactions: The SN 1 Mechanism 154
4.10 Structure, Bonding, and Stability of Carbocations 155
4.11 Effect of Alcohol Structure on Reaction Rate 158
4.12 Reaction of Methyl and Primary Alcohols with Hydrogen Halides: The SN 2
Mechanism 159
4.13 Other Methods for Converting Alcohols to Alkyl Halides 160
4.14 Halogenation of Alkanes 161
4.15 Chlorination of Methane 162
4.16 Structure and Stability of Free Radicals 162
4.17 Mechanism of Methane Chlorination 167
4.18 Halogenation of Higher Alkanes 168
From Bond Energies to Heats of Reaction 169
4.19 Summary 173
Alkenes (Elimination Reactions)
Structure and Preparation of Alkenes: Elimination
Reactions 182
5.1 Alkene Nomenclature 183
5.2 Structure and Bonding in Alkenes 185
Ethylene 186
5.3 Isomerism in Alkenes 187
5.4 Naming Stereoisomeric Alkenes by the E–Z Notational System 188
5.5 Physical Properties of Alkenes 189
5.6 Relative Stabilities of Alkenes 191
5.7 Cycloalkenes 195
5.8 Preparation of Alkenes: Elimination Reactions 196
5.9 Dehydration of Alcohols 197
5.10 Regioselectivity in Alcohol Dehydration: The Zaitsev Rule 198
5.11 Stereoselectivity in Alcohol Dehydration 199
5.12 The E1 and E2 Mechanisms of Alcohol Dehydration 200
5.13 Rearrangements in Alcohol Dehydration 202
5.14 Dehydrohalogenation of Alkyl Halides 205
5.15 The E2 Mechanism of Dehydrohalogenation of Alkyl Halides 207
5.16 Anti Elimination in E2 Reactions: Stereoelectronic Effects 209
5.17 Isotope Effects and the E2 Mechanism 210
5.18 The E1 Mechanism of Dehydrohalogenation of Alkyl Halides 211
5.19 Summary 213
6. Alkenes (Addition Reactions)
Addition Reactions of Alkenes 224
6.1 Hydrogenation of Alkenes 225
6.2 Heats of Hydrogenation 226
Stereochemistry PART 2
6.3 Stereochemistry of Alkene Hydrogenation 229
6.4 Electrophilic Addition of Hydrogen Halides to Alkenes 229
6.5 Regioselectivity of Hydrogen Halide Addition: Markovnikov’s Rule
231
6.6 Mechanistic Basis for Markovnikov’s Rule 233
Rules, Laws, Theories, and the Scientific Method 235
6.7 Carbocation Rearrangements in Hydrogen Halide Addition to Alkenes
235
6.8 Free-Radical Addition of Hydrogen Bromide to Alkenes 236
6.9 Addition of Sulfuric Acid to Alkenes 239
6.10 Acid-Catalyzed Hydration of Alkenes 241
6.11 Thermodynamics of Addition–Elimination Equilibria 243
6.12 Hydroboration–Oxidation of Alkenes 246
6.13
Stereochemistry of Hydroboration–Oxidation
248
6.14 Mechanism of Hydroboration–Oxidation 248
6.15 Addition of Halogens to Alkenes 251
6.16 Stereochemistry of Halogen Addition 251
6.17 Mechanism of Halogen Addition to Alkenes: Halonium Ions 252
6.18 Conversion of Alkenes to Vicinal Halohydrins 254
6.19 Epoxidation of Alkenes 255
6.20 Ozonolysis of Alkenes 257
6.21 Introduction to Organic Chemical Synthesis 259
6.22 Reactions of Alkenes with Alkenes: Polymerization 260
Ethylene and Propene: The Most Important Industrial Organic Chemicals 265
6.23 Summary 266
7. Stereochemistry PART
1:
Stereochemistry 276
7.1 Molecular Chirality: Enantiomers 277
7.2 The Chirality Center 279
7.3 Symmetry in Achiral Structures 281
7.4 Optical Activity 282
7.5 Absolute and Relative Configuration 284
7.6 The Cahn–Ingold–Prelog R–S Notational System 285
7.7 Fischer Projections 288
7.8 Properties of Enantiomers 290
Chiral Drugs 291
Stereochemistry PART
2:
7.9 Reactions That Create a Chirality Center 292
7.10 Chiral Molecules with Two Chirality Centers 295
7.11 Achiral Molecules with Two Chirality Centers 297
7.12 Molecules with Multiple Chirality Centers 299
Chirality of Disubstituted Cyclohexanes 300
7.13 Reactions That Produce Diastereomers 301
7.14 Resolution of Enantiomers 303
7.15 Stereoregular Polymers 305
7.16 Chirality Centers Other Than Carbon 306
7.17 Summary 307
Nucleophilic Substitution 318
8.1 Functional Group Transformation by Nucleophilic Substitution 319
8.2 Relative Reactivity of Halide Leaving Groups 322
8.3 The SN 2 Mechanism of Nucleophilic Substitution 323
8.4 Steric Effects in SN 2 Reaction Rates 326
8.5 Nucleophiles and Nucleophilicity 328
8.6 The SN 1 Mechanism of Nucleophilic Substitution 330
Enzyme-Catalyzed Nucleophilic Substitutions of Alkyl Halides 331
8.7 Carbocation Stability and SN 1 Reaction Rates 331
8.8 Stereochemistry of SN 1 Reactions 334
8.9 Carbocation Rearrangements in SN 1 Reactions 335
8.10 Effect of Solvent on the Rate of Nucleophilic Substitution 337
8.11 Substitution and Elimination as Competing Reactions 339
8.12 Nucleophilic Substitution of Alkyl Sulfonates 342
8.13 Looking Back: Reactions of Alcohols with Hydrogen Halides 344
8.14 Summary 346
Alkynes 354
9.1 Sources of Alkynes 355
9.2 Nomenclature 357
9.3 Physical Properties of Alkynes 357
9.4 Structure and Bonding in Alkynes: sp Hybridization 357
9.5 Acidity of Acetylene and Terminal Alkynes 360
9.6 Preparation of Alkynes by Alkyation of Acetylene and Terminal Alkynes 361
9.7 Preparation of Alkynes by Elimination Reactions 363
9.8 Reactions of Alkynes 364
9.9 Hydrogenation of Alkynes 365
9.10 Metal–Ammonia Reduction of Alkynes 367
9.11 Addition of Hydrogen Halides to Alkynes 368
9.12 Hydration of Alkynes 370
9.13 Addition of Halogens to Alkynes 371
Some Things That Can Be Made from Acetylene...But Aren't 372
9.14 Ozonolysis of Alkynes 372
9.15 Summary 373
CHAPTER 10
Conjugation in Alkadienes and Allylic Systems 382
10.1 The Allyl Group 383
10.2 Allylic Carbocations 384
10.3 SN 1 Reactions of Allylic Halides 385
10.4 SN 2 Reactions of Allylic Halides 388
10.5 Allylic Free Radicals 389
10.6 Allylic Halogenation 390
10.7 Allylic Anions 393
10.8 Classes of Dienes 394
10.9 Relative Stabilities of Dienes 395
10.10 Bonding in Conjugated Dienes 396
10.11 Bonding in Allenes 398
10.12 Preparation of Dienes 399
10.13 Addition of Hydrogen Halides to Conjugated Dienes 400
10.14 Halogen Addition to Dienes 403
10.15 The Diels–Alder Reaction 403
Diene Polymers 404
10.16 The Pi Molecular Orbitals of Ethylene and 1,3-Butadiene 407
10.17 A Pi Molecular Orbital Analysis of the Diels–Alder Reaction 408
10.18 Summary 410
CHAPTER 14
Organometallic Compounds 578
14.1 Organometallic Nomenclature 580
14.2 Carbon–Metal Bonds in Organometallic Compounds
580
14.3 Preparation of Organolithium Compounds 581
14.4 Preparation of Organomagnesium Compounds: Grignard Reagents 583
14.5 Organolithium and Organomagnesium Compounds as Brønsted Bases 584
14.6 Synthesis of Alcohols Using Grignard Reagents 586
14.7 Synthesis of Alcohols Using Organolithium Reagents 588
14.8 Synthesis of Acetylenic Alcohols 588
14.9 Retrosynthetic Analysis 589
14.10 Preparation of Tertiary Alcohols from Esters and Grignard Reagents 592
14.11 Alkane Synthesis Using Organocopper Reagents 593
14.12 An Organozinc Reagent for Cyclopropane Synthesis 595
14.13 Carbenes and Carbenoids 596
14.14 Transition-Metal Organometallic Compounds 599
An Organometallic Compound That Occurs Naturally: Coenzyme B12 601
14.15 Homogeneous Catalytic Hydrogenation 602
14.16 Olefin Metathesis 605
14.17 Ziegler–Natta Catalysis of Alkene Polymerization 607
14.18 Summary 610
Problems 613
Descriptive Passage and Interpretive Problems 14: Oxymercuration 617
CHAPTER 15
Alcohols, Diols, and Thiols 620
15.1 Sources of Alcohols 621
15.2 Preparation of Alcohols by Reduction of Aldehydes and Ketones 622
15.3 Preparation of Alcohols by Reduction of Carboxylic Acids and Esters 628
15.4 Preparation of Alcohols from Epoxides 629
15.5 Preparation of Diols 630
15.6 Reactions of Alcohols: A Review and a Preview 632
15.7 Conversion of Alcohols to Ethers 632
15.8 Esterification 635
15.9 Esters of Inorganic Acids 637
15.10 Oxidation of Alcohols 638
15.11 Biological Oxidation of Alcohols 640
Economic and Environmental Factors in Organic Synthesis 641
15.12 Oxidative Cleavage of Vicinal Diols 643
15.13 Thiols 644
15.14 Spectroscopic Analysis of Alcohols and Thiols 647
15.15 Summary 648
Problems 652
Decriptive Passage and Interpretive Problems 15: The Pinacol rearrangement 658
Selected Topics:
14. Organometallics
15.
Alcohols, Diols, and Thiols
(QuizSolutions)
(QuizSolutions)
Worksheets: (Collaborative/Group)
NOTE: Worksheets are in pdf format. You will need Adobe Acrobat Reader
to view and print them which can be downloaded for free at: http://www.adobe.com/products/acrobat/readstep2.html
Chemical
Bonding Concept Map .pdf
Organic
Molecules
(1) .pdf
(Bonds, Stuctures, Formulas & Shapes)
Related Web-page: http://chemconnections.org/organic/chem226/Labs/VSEPR/
Organic
Molecules (2) .pdf (Stuctures,
Formulas & Orbitals)
Organic
Molecules (3) .pdf (Organic
Functional Groups)
Organic
Molecules
(4) .pdf
(Functions, Polarity, Formal Charge)
Organic
Molecules (5) .pdf (Acids &
Bases) Charge
Table .pdf; Charge
Table .html
Alkane/Alkene
Worksheet (6)
.pdf
Refer to:
Hydrocarbon Stabilities
/ Isomerism: Value for your gasoline dollar.
Conformational Analysis Worksheet (7) Part
I .pdf; Part
II .pdf
Conformational &
Structural Exercises based on Computational Chemistry
Reactions of Alkenes Worksheet
(8) .pdf
Stereochemistry
I (9).pdf
Stereochemistry
II (10) .pdf
Free
Radical Reactions/ Stereochemistry .pdf
Product 1 - Product
2
Alkynes: reactions,
reagents, synthesis (11) .pdf
Diels
Alder .pdf
Molecular
Modeling: Diels Alder Reactions (I) .pdf
Molecular
Modeling: Diels Alder Reactions (II) .pdf
Halides-Tosylates I .pdf
Halides-Tosylates II .pdf
Halides-Tosylates Synthesis .pdf
Molecular
Modeling / Resonance .pdf
Class Assignments
Review .pdf
Laboratory:
- ORGANIC
COMPOUNDS: Preparation / Isolation /
Purification / Identification;
- SAFETY: General Regulations & Lab Guidelines
Safety
Quiz Sheet & Acknowledgment
- LABORATORY EQUIPMENT: Aldrich-Kit with Ground Glass Joints
- LABORATORY SKILLS: Organic
Lab Skills & Operations Check List
- RECORD KEEPING:
Research Notebooks & Reports
NOTE: Check
List must be completed as
part of the pre-lab before you may start any lab experiment. You should be
able to describe each skill that is to be used in the lab to Dr. R..
Upon
completion
of the lab experiment you
should be
able to demonstrate and to teach someone the skills that you
acquired. Before beginning the first lab experiment, you are to review
the Lab Text/Guide's Table
of Contents relative to the check list and provide the appropriate page
numbers for the respective Skill/Operation from the text and your comments
on how understandable and complete the text's information is to you.
Complete
the form and
turn-in
to Dr.
R.
Tentative Lab Schedule: (Refer
to the course calendar for more exact details and for Due
dates.)
| Skills & Operations:
|
Exercises / Activities /
Experiments
|
|
1. Use & Care of Tapered Glassware.
2. Weighing Techniques: Tare & Care
3. Transferring Liquids.
4. Care, Handling & Storage of Chemicals.
5. Chemical Hygiene & Waste Disposal
6. Temperature: Measurement & Control.
7. Heating Methods.
8. Reflux.
9. Cooling Methods.
10. Methods of Addition (s, l, & g).
11. Filtration (Gravity).
12. Filtration (Vacuum/Aspirator).
13. Extraction.
14. Evaporation.
15. Rotevap: Recovery of Solvents.
16. Column Chromatography.
17. Thin-Layer Chromatography.
18. Gas Chromatography.
19. Washing Liquids.
20. Drying Liquids.
21. Drying Solids.
22. Drying and Trapping Gases.
23. Recrystallization.
24. Sublimation.
25. Steam Distillation.
26. Simple Distillation.
27. Vacuum Distillation.
28. Fractional Distillation.
29. Melting Point Determination.
30. Boiling Point Determination.
31. Refractive Index Determination.
32. Polarimetry: Optical Rotation.
33. IR: Infrared Spectrometry.
34. NMR: Nuclear Magnetic Resonance.
35. Ultraviolet-Visible Spectrometry.
36. Mass Spectrometry.
|
Learning Styles Survey:
Organic Chemistry / Memory / Learning
Safety:
SAFETY: General
Regulations & Lab Guidelines
Safety
Quiz Sheet & Acknowledgment
MSDS/ Organic Chemistry:Toxicity,
Health & Safety (pdf
files)
Part I: Definitions (Individual)
Part II:: MSDS
Data acquisition, interpretation, application & communication (Group)
NFPA Hazard Classifications
& Placard
Molecular Modeling
I / WebMO / Dipole Moment
Part 1; Part
2
Smell / Olfaction: (pdf files)
Part
I: Odor and Molecules/ Distinguishing Organic Molecules
Based on Odor
Michael Rossman: A case of anosmia easing incarceration
Part II: Odor and Molecular Formulas
Part III: Odor and Functionality
Part IV: Chemical Communication
Chemical Communication html : http://chemconnections.org/organic/chem226/Labs/Smell/ChemComm.html
Smell & Stereochemistry
html:
http://chemconnections.org/organic/chem226/Labs/Smell/Smell-Stereochem.html
Molecular Modeling II:
Conformations
Molecular Modeling IIa:
Cyclic / Acyclic Hydrocarbons:Material Properties
Experiment #1 (demo): Extraction (Observed)
Experiment #1: Extraction
Experiment
#2: Recrystallization & Melting
Point
Experiment #3: Thin
Layer Chromatography (TLC)
TLC of Analgesic Drug Components
Experiment #4: Synthesis of Salicylic Acid
from Wintergreen
Experiment
#5: Enantiomeric Separation/ Resolution (Ibuprofen)
Optical Activity /Polarimetry: Part
I & Part II
Optical
Rotation I .pdf; Optical
Rotation II .pdf; Optical
Rotation III .pdf
Experiment #6: Gas Chromatography & Fractional
Distillation
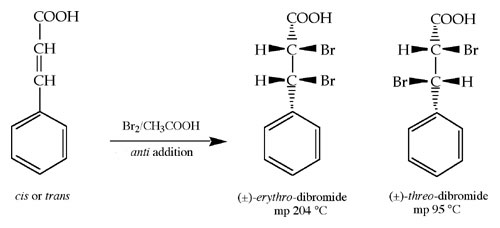
Experiment #7: Bromination of Cinnamic Acid
Experiment #8: Acetate Synthesis, Simple
Distillation, Infrared Spectroscopy, GC
Experiment #9: Sn1 ,
Sn2 Reactions and Solvent Effects
Experiment #10: Essential Oils / Steam Distillation
/ Extraction
Experiment #11: Diels
Alder reaction of maleic anhydride and furan
Experiment #12: Identification
of Terpenoids
Experiment #13 : Colorful
Grignard Reaction
|
| |
|
Laboratory
Techniques & Videos:
Laboratory Experiments
These experiments have been adapted from standard types of experiments
commonly performed in organic chemistry courses throughout North America.
They are derived from the non-copyrighted, open Web source materials kindly
made available by a large number of notable educators/professors who generously
share their creativity, time, efforts,
and excellent materials. Their institutions include: University of Colorado,
Boulder; McMaster University; University of Alberta; University of Calgary;
Reed College, Barnard University; Massachusettes Institute of Technology;
University of California, Los Angeles;
Dakota State University; Wellesley University;
University of California, Berkeley; Mount Holyoke College, Manhattan College.
 |
- Solubility / Extraction / Separation (Observed)
Applying solubility differences in immiscible solvents:
(DEMO) The relative distribution of I2 in
a water layer versus an organic solvent layer (Methylene chloride: CH2Cl2)
is examined.
- This type of solubility difference is the basis for the fundamental
way organic chemicals are separated.
|

|
Extraction: Isolation
/ Separation of 4-Chloroaniline (p-chloroaniline), Benzoic
acid,
and
4-Dibromobenzene (p-dibromobenzene)
Prelab questions
See:
Introduction
Procedure / Instructions
Background & Tutorial:
http://www.chem.ualberta.ca/%7Eorglabs/Interactive%20Tutorials/separation/mastercopy/Extraction.htm
Filtration & Liquid/Liquid Extraction:(Videos)
http://www.oid.ucla.edu/Webcast/chemistry/index.html
http://ocw.mit.edu/ans7870/resources/chemvideo/index.htm (Reaction
Work-Up I)
http://www.chem.ualberta.ca/%7Eorglabs/Techniques.html
Post Lab Questions:
- Briefly explain why sodium benzoate is more soluble in H2O
rather than in ether, but its conjugate acid is more soluble in ether
rather than H2O.
- The solubility of p-cresol in water at 25 oC is 56.0 g/L and in
ether at the same temperature 310.0 g/L. (a) Calculate
the value of Kp. (b) For a solution of 2.5 g of
p-cresol in 50 mL of water,
calculate
the
weight of p-cresol that would be extracted into 150
mL of ether
by (1) a single extraction, and (2) the total weight
extracted by three sequential extractions with 50 mL of ether
each.
- Using extraction and the reagents and solvents from question
#3 of the prelab, show a separation scheme with line drawings
for the compounds, the appropriate reagents for each step and the solvent
used to separate a four component mixture of phenylacetic acid (pKa
= 4.28), o-cresol
( pKa
= 10.2),
o-xylene
(a neutral compound,) and quinoline, a base (pKa of quinoline's conjugate
acid = 4.90)?
Grading Rubrik
Results 2009
Recrystallization: Purification
of
an Unknown Solid
See:
Introduction
Procedure / Instructions
Background:
http://ocw.mit.edu/ans7870/resources/chemvideo/index.htm
http://www.oid.ucla.edu/Webcast/chemistry/index.html
http://www.chem.ualberta.ca/%7Eorglabs/Techniques.html
Melting Point:
Identification of
an Unknown Solid
See:
Introduction
Procedure / Instructions
Background:
http://ocw.mit.edu/ans7870/resources/chemvideo/index.htm
http://www.oid.ucla.edu/Webcast/chemistry/index.html
Post Lab Questions:
- Turn-in your sample clearly labeled with your name and pertinent
information as per the Lab instructions.
- Benzoic acid is one component in a two component mixture. The other
component is either ortho
toluic acid, phenyl succinate
or meta
aminophenol. Mixed melting points were done with benzoic acid plus
each of the three possibilities. The mixture with ortho toluic acid
began
to melt at 89oC. The mixture with phenyl succinate
began
to melt at 120oC. The mixture with meta
aminophenol
began
to melt at 102oC. The melting points of the pure compounds are: benzoic
acid =121oC, ortho
toluic acid = 102 oC, phenyl succinate = 121 oC,
and meta
aminophenol = 122oC. What is the second compound in the mixture?
Explain how you decided.
- What properties are necessary and desirable for a single solvent
in order that it be well suited for recrystallizing a particular
organic compound?
- Which of the following mixtures could not be used
for two-solvent systems and why?
(a) acetone-water (b) dichloromethane-water (c) ethanol-water
(d) ether-water (e) hexane-water (f) toluene-water
- Suppose that
your sample had contained black colored impurities.
How would you modify the recrystallization
procedure
to provide easy and efficient decolorizing of the sample?
- Why
is suction filtration preferable to ordinary gravity filtration
for separating punfied crystals from the mother liquor? Why should
the vacuum be released at the apparatus before turning off
the
aspirator?
-
- Grading Rubrik
Synthesis
of Salicylic Acid from Wintergreen Oil-
Experimental
Introduction
Procedure / Instructions
Complete Post lab questions:
- 1. What happened to the methanol produced in the reaction?
2. If you calculated that you needed 10 mL of 3M sulfuric acid but you discover
that the only acid available is 6M hydrochloric acid, how many mL would you
use?
3.. If the entire salicylic acid sample that you obtained were reacted to produce
a 100% yield of aspirin, how much aspirin (mg) would be produced?
- (Read the following and answer the related questions):
- http://chemconnections.llnl.gov/Organic/Chem226/Announcements-info/aspirin-CEN-8-18-1997.html
- http://chemconnections.llnl.gov/Organic/Chem226/Announcements-info/aspirin-econ-8-9-1997.html
a) Who discovered the structure and first synthesized salicylic acid?
In what year?
- b) Who was awarded a Nobel prize for determining how aspirin works?
In what year were the first clinical trials done?
c) If there were 250 mg of aspirin in every tablet sold, how many metric
tons of aspirin would be produced each year? Show your calculation.
d) Provide a generic name for the local hormones which are affected
by aspirin; draw a structure for one of them and name it.
e) Explain the relationship of cyclooxygenase (cox) enzymes to
analgesic acitivty. What are the diferences between
the enzymes cox-1 and cox-2? What may cox-3 be involved with? What
is different about "new" improved Tylenol Extra.
Why do you think that Johnson & Johnson developed
it?
Acetate
Synthesis, Simple Distillation, Infrared Spectroscopy, Index of refraction, [GC]
A) Complete the prelab form using the links
below to find which ester that you are to synthesize.
Pre-lab: Click on the
images or letters below to see which acetate ester.corresponds to
each smell. The letters A and B
are two different possiblities..
Pre lab questions & form
To discover the ester that you are to synthesize click
here to
find the randomly assigned fruity smell that it relates to;
then complete the pre-lab questions. For questions #2 and #3 choose
either A or B for
banana and pear respectively, but, if
an A or B follows your assigned smell
be sure to use that corresponding ester for
the pre-lab questions #2 and
#3. Refer to: Acetate Boiling Point Table
Experimental
Introduction
Procedure /
Instructions
Prepare
your lab
notebook using the correct alcohol for the assigned smell, include
its physical and chemical properties in the data report table for
your prelab. Calculate the appropriate amounts of alcohol
and glacial
acetic acid. Neatly sketch the apparatus (assembly) that you plan
to use for the reaction in your notebook.Complete the lab skills
check
list and then have Dr. R. initial your notebook.
Begin and complete the synthesis after pre lab approval. Record
the mass of crude product and calculate the % yield. Run an IR
on the crude product.
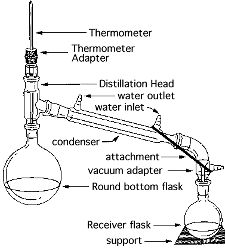
B) Distill crude product. (Simple
distillation.)
Simple Distillation:
http://ocw.mit.edu/ans7870/resources/chemvideo/index.htm
C) Identify functions from infrared spectra
1) Refer to IR-Tutor & tutorial: http://wwwchem.csustan.edu/Tutorials/INFRARED.HTM
2) Begin the worksheet, Infrared
Analysis (IR) / Synthesis of Acetates .pdf : Unknown
spectra (Identify the chemical function present in
each Web unknown assigned to you. If your DVC ID ends
in an odd number, do the
odd Unknowns; if it ends in an even number or zero, do the even.
Then, find a partner who has done the other set and explain your
assignments
of functions and peaks in the spectra that support your choices. Complete
the entire form of 10 unknowns. The selection is limited
to alcohols,
carboxylic acids, esters, ethers, ketones and aldehydes. Be sure
to provide the key peak(s) in the spectrum that support your assignment
of the
function. [NOTE: The molecules contain only C,H,O; there are no nitrogen
atoms in the molecules.]
3) Run FT-IR spectra on 1) your individual unknown liquid
and 2) the ester that you synthesized and distilled.
4) Analyze the spectra. 1. What function is present in the
liquid unknown? 2. Consider the starting alcohol and glacial
acetic acid: are either or both of them present
as impurities in your product? What peaks indicate the presence of
product (ester)? Are the peaks
in either
of the reactants?
Complete the form: Infrared
Analysis (IR) / Synthesis of Acetates .pdf
IR Analysis: (Works
only with Windows PCs and Chime: PS 110 Computers are OK.) http://www.umass.edu/microbio/chime/ir-spect/
D) Determine the product's index of refraction.
(Include in lab report with the boiling point of your product.)

Click on the image above for instrument
instructions.
E) Analyze Product by GC.
(Optional as time and GC availability allows. If completed, include in
lab report with calculation of the % purity of your product.)
Post lab questions:
a) Major League Baseball & Chewing Gum
b) Complete Odor,
Functions & Structures .pdf (Post
lab); refer to the review article on reserve in the DVC library.
"Structure-Odor Relationships", Karen J. Rossiter,
Chem. Rev., 96, 3201-3240, 1996 (Library Reserve under Dr. R's
name.) Reading: pp. 3201-08, 3216-26.
[For a better view of the rose oxide isomers see: rose
oxide #1 and
rose oxide #2. Rose oxide #1 is ~100x more powerful in its scent than
#2.]
- Gas Chromatography & Fractional
Distillation
- Experimental
Introduction
Procedure / Instructions
VIDEOS:
Gas Cromatography:
http://www.oid.ucla.edu/Webcast/chemistry/index.html
- Simple & Fractional Distillation:
- http://ocw.mit.edu/ans7870/resources/chemvideo/index.htm
http://www.oid.ucla.edu/Webcast/chemistry/index.html
-
-
-
- General instructions .pdf
Prelab Form .pdf
Pre lab
questions .html
Separations Class
Separations ppt
Separations pdf
-
- Unkown Retention Times
Logger Pro Example
GC Liquid-Stationary Phases
GC Fractional Distillation
Data (Option: you can use your
data or this set for graphing.)
The fractionating columns below are a packed column
on the left and a Vigreaux column on the right.

|
 |
Post lab QUESTIONS:
- 1) Recommend a stationary phase, column temperature and
helium flow rate to analyze an ester like banana oil, isoamyl
acetate.
Read the following and answer the related questions #s 2-4.
NYT-auto-smell.html
NYT-Sniffing-Cars.html
VOCs-C&EN.html
2) The media ascribes new car smell to a single compound which
is used to keep plastics soft and is also attributed to
leaving a film on the inside of car windows (fogging). The compound
is
bis (2-ethylhexyl) phthalate. Briefly explain why
the media is likely wrong.
3) Australian researchers at CSIRO detected 30-40 volatile
organic compounds (VOCs) in new car smell.
a) What analytical method did they use?
b) What are the symptoms of "negative sensory effects"?
c) In the study, what % of the VOCs remain in a new car after 6
months?....after 2 years?
4) New car smell can be purchased at auto supply stores
as a "household"
product. Manufacturers refuse to diviluge the contents,
but a fragrance industry expert believes that it is a mixture
of compounds respectively
with three different chemical functions.
a) Name the three functions.
b) Referring to the chart of functional affinities for various
GC stationary phases, select a single stationary phase for the
separation of the 3 types of functions and rank the expected order
of their relative retention times from lowest to highest.
5) From the article GC
and the Coffee Crisis (pdf):
a) Draw a condensed structure for the compound found in the
GC of coffee that causes it to taste sour after heat processing.
b) Name the three types of heterocylic compounds that are antioxidants
which are found in coffee. Identify those that contain nitrogen
and those that contain oxygen in their molecular formulas
GRADING Rubrik
-
Essential
Oils / Steam Distillation / Extraction
Experimental
Introduction
Procedure / Instructions
IR spectra (clove oil components): major
component ; minor component
(~10%)
Post Lab Questions:
- Draw the structure of eugenol. Circle and label the chemical functions.
- On your IR: clearly identify and label the peaks that correspond
to the functions cited above.
- Draw a separation scheme [organic layer (dichloromethane) vs. aqueous
layer] that shows how eugenol was recovered from the distillate using
NaOH and HCl. Use chemical equations and arrows to indicate electron
movement for each step.
- Clove oil contains a minor component that has the formula C12H14O3.
It produces eugenol and acetic acid upon acid catalyzed hydrolysis
similar to methylsalicylate-salicylic acid. Draw a structure for the
compound. Analyze your IR for its presence. Explain whether it is there
or not from the IR data.
- Clove oil contains a structurally interesting isoprenoid in relatively
small amounts. Its IUPAC name is (E)-4,11,11-trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene.
What is the common name for this compound? Draw a structure for the
compound. How many isoprene units are in the compound? Which class
of terpene is it in?.... monoterpene, sesquiterpene, diterpene, sesterpene,
triterpene, tetraterpene.
IR spectra (cumin extract): major component ; minor component
Structures of components
Post Lab Questions:
- Draw the respective structures of the major and inor components of
cumin. Circle and label the chemical functions in each.
- From their respective IRs: clearly identify and label the peaks that
correspond to the functions cited above.
- Briefly explain why the base-acid extractions that were done with
eugenol and clove oil do not work to separate and purify cumin.
Answer questions 4, and 5 above for eugenol and clove oil:
Clove oil
contains a minor component that has the formula C12H14O3.
It produces eugenol and acetic acid upon acid catalyzed hydrolysis
similar to methylsalicylate-salicylic acid. Draw a structure for
the compound. Analyze your IR for its presence. Explain whether it
is there
or not from the IR data.
Clove oil contains a structurally interesting isoprenoid in relatively
small amounts. Its IUPAC name is (E)-4,11,11-trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene.
What is the common name for this compound? Draw a structure for the
compound. How many isoprene units are in the compound? Which class
of terpene is it in?.... monoterpene, sesquiterpene, diterpene, sesterpene,
triterpene, tetraterpene.
Identification
of Terpenes
Terpenes/
UV Absorbtion Class
Terpenes/ UV
Absorbtion pdf

Experimental
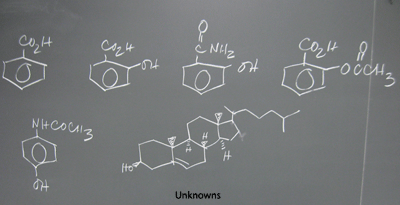
Unknown# ASSIGNMENTS
Unknown DATA
UV Analysis Table
UV Analysis
Table .pdf
Thin Layer Chromatography
(TLC)
MOVIES:
Thin Layer Chromatography (TLC):
http://www.oid.ucla.edu/Webcast/chemistry/index.html
http://ocw.mit.edu/ans7870/resources/chemvideo/index.htm
http://www.chem.ualberta.ca/%7Eorglabs/Techniques.html
Rf values: http://orgchem.colorado.edu/hndbksupport/TLC/TLCrf.html

Polarimetry
/ Stereochemistry/ Optical Resolution
Stereochemistry
& Polarimetry
Part 1: Optical Rotation/ Carvone
Optical Rotation
/ Polarimetry: PART I .pdf
Part 2: Chiral
Molecules: Structure & Configuration
Optical Rotation:
PART II.pdf
Part 3: Enantiomeric Resolution of (+/-) Ibuprofen
Powerpoint
Outline class; Optical
Rotation: PART III.pdf
Bromination of Cinnamic Acid
Prelab Worksheet
Experimental
Introduction
Procedure / Instructions
SN1 ,
SN2 Reactions and Solvent Effects

Part 1: Individually, CPR-
Calibrated Peer Review
Part 2: Team experimentation, analysis and presentations. (Handouts)
Procedures
.pdf
Infection
/ Bacterial Resistance / Nucleophilic Displacement
Calvin
Coolidge, Bacteria & Fate
"Shortly after the 1924 Republican Party convention, President
Calvin Coolidge experienced a personal tragedy. Coolidge's younger
son,
Calvin, Jr.,
developed
a blister
from playing
tennis on the White House courts. The blister became infected, and
Calvin, Jr. died. After that Coolidge became even more withdrawn.
He later said that "when he died, the power and glory of the
Presidency went with him."
Reading:
Science-SN2-description.html
Science-SN2-article.html
Supplemental:
1) The "Roundabout" SN2 Mechanism- C&EN-08-description .pdf
2) The "Roundabout" SN2 Mechanism- Science-08-article
.pdf
- Diels Alder Reactions / Molecular Modeling
Introduction pdf

Experimental Procedure pdf

Molecular Modeling
I pdf
Molecular Modeling
II pdf
Post Lab
Questions pdf
-
- Lehman Experiment MINILAB #27: Diels
Alder reaction of maleic anhydride and furan
University of Saskatchewan Alternative
procedure .pdf
Molecular Modeling: Diels
Alder Reactions (I) .pdf
Molecular Modeling: Diels
Alder Reactions (II) .pdf
-
- Colorful
Grignard (14/20) (19/22)
|
 |
Click on image for larger file

|
Crystal
Violet & Malachite
Green (pdf handouts)
Assigned Reagent
Vision Class
Vision pdf
Pre- and Post Lab: Answer questions from handout.
Lehman Experiment #32:Conjugated
Diene / Diels Alder Reaction
Questions 1, 2, 3 & 5
Triphenylmethanol IR: KBr, nujpl mull respectively
http://www.aist.go.jp/RIODB/SDBS/ir/NIDA63390.gif
http://www.aist.go.jp/RIODB/SDBS/ir/NIDA64310.gif
On-line End-of-course
Evaluation
Course ID : 13802
Password: chem226
![]() Webassign
Homework .
All assignments are to be done individually. Due dates are embedded on line
and these homework problems should be your main focus along with the collaborative/group
Worksheets.
Webassign
Homework .
All assignments are to be done individually. Due dates are embedded on line
and these homework problems should be your main focus along with the collaborative/group
Worksheets.