Laboratory Techniques & Videos:
Exercises / Worksheets / Tutorials:
- Spectroscopy for the Organic Chemistry Student from Professor Paul R. Young: University of Illinois, Chicago Organic Chemistry OnLine: Spectroscopy
- Section VIa (IR Background): Infrared Spectroscopy
- Tutorial Problem Set ( Ten Problems):
- Infrared Spectroscopy Problem Set
- Spectroscopy for the Organic Chemistry Student from Professor Paul R. Young: University of Illinois, Chicago Organic Chemistry OnLine: Spectroscopy
- Section VIc (Background): Mass Spectroscopy
- Tutorial Problem Set (Five Problems)
- Mass Spectroscopy Problem Set
- SDBS Mass Spectrometry Database A collection of spectra for over 200 relatively simple organic compounds containing up to 30 carbon atoms
- Identify the protons as equivalent or diastereotopic or enantiotopic.
- Predict the chemical shifts and mutliplicities.
- Draw the spectrum.
- Compare to example.
1H nmr data for your individual unknown is to be generated using the department's 60 MHz FT-NMR spectrometer.
- Identify the carbons as equivalent or different.
- Predict the chemical shifts and mutliplicities. (Decoupled)
- Draw the spectrum.
- Compare to example.
13C nmr data for your individual unknown is to be generated using the department's 60 MHz FT-NMR spectrometer.
13C nmr: Worksheet spectrum - Spectroscopy for the Organic Chemistry Student from Professor Paul R. Young: University of Illinois, Chicago Organic Chemistry OnLine: Spectroscopy
- Section VIb Background: 1H & 13C NMR
- Tutorial Problem Sets (Ten 1H & Five 13C Problems)
- 1H & 13C NMR Problem Set
- NMRs of 1- and 3-methyl cyclopentene and their mixture.
 NMR
QuickTime Movie (156Kb)
NMR
QuickTime Movie (156Kb)
Spectroscopy 1: IR Spectroscopy: Handout [Group] & [Individual]
IR (Infrared) / UV Spectrosopy & Mass Spectrometry
Worksheet FORM: IR (Infrared) / UV Spectrosopy & Mass Spectrometry (MS) Lab Worksheet .pdf(MS data for the individual unknowns is provided by Dr. R. in response to your e-mail reply to the assignment from first class meeting.)

IR Resources:
Infrared Basic Tutorial with six practice problems California State University, Stanislaus
IR Wizard from Steffan (St.) Thomas, University of Potsdam, Germany. A query based tool in English and German. Useful in self-learning-testing applications. Supply a wave number (cm-1) and a table of possibilities is produced with chemical functions that have the value within their range of peak frequencies. Pages are cleanly designed and the built-in search engine works well.
NIST Chemistry Webbook
http://webbook.nist.gov/chemistry
Free government database that includes numerous compounds. Provides
a variety of data: physical, thermodynamic and spectroscopic. IR and MS
spectra available for a portion of the compounds in the data base. An
excellent resource.
Spectral Data Base System
for Organic Compounds
http://www.aist.go.jp/RIODB/SDBS/
SDBS: Integrated Spectral Data Base System for Organic Compounds, a searchable
database that contains: MS (ca 18,000 spectra), 13C NMR (ca 9,700 spectra),
Compound Dictionary, 1H NMR (ca 10,100 spectra, 29,000 compounds) add
IR and Raman.
![]()
![]() Interactive
Tutorial: University of Alberta
Interactive
Tutorial: University of Alberta
http://www.chem.ualberta.ca/~orglabs/spectroscopy/specmaster.html
An excellent resource for practice in prediction and interpretation.
Mass Spectrometry:
Mass Spectrometer Animated Tutorial Dr. Thomas Poon, Colby College mass spectrometer Shockwave Interactive Tutorial: Instrument Function, Data Generation, Intrepretation and More. (127 kB)
MS Fragment
Wizard from Steffan (St.) Thomas, University of Potsdam, Germany.
A query based tool in English and German. Useful in self-learning-testing
applications. Supply m/e value and a list of possibilities for the
fragment or mass loss fragment is produced with links to exact mass and
isotope distribution calculators. Pages are cleanly designed and
the built-in search engine works well.
NIST Chemistry Webbook
http://webbook.nist.gov/chemistry
Free government database that includes numerous compounds. Provides
a variety of data: physical, thermodynamic and spectroscopic. IR and MS
spectra available for a portion of the compounds in the data base. An
excellent resource.
MRI Photos
http://www.simplyphysics.com/flying_objects.html
Spectroscopy 2: 1H NMR Spectroscopy
1H NMR Spectrosopy
Worksheet Form: 1H NMR Spectrosopy: Interpretation / Prediction & Reactions Lab Worksheet .pdf
Interactive Tutorial: University of Alberta
http://www.chem.ualberta.ca/~orglabs/spectroscopy/specmaster.html
An excellent resource for practice in prediction and interpretation.
The Basics of NMR: Dr. Joseph Hornak http://www.cis.rit.edu/htbooks/nmr/nmr-main.htm
1 H and 13C NMRs :
Example compound: 1H nmr - example

Prediction & Interpretation: (Could also be Interpretation and Comparison.)
Spectroscopy 3: 13C NMR Spectroscopy
13C NMR Spectrosopy
Worksheet Form: 13C NMR Spectrosopy: Interpretation / Prediction & Reactions Lab Worksheet .pdf
The Basics of NMR: Dr. Joseph Hornak http://www.cis.rit.edu/htbooks/nmr/nmr-main.htm
1 H and 13C NMRs :
Example compound: 13C nmr - example

Prediction & Interpretation: (Could also be Interpretation and Comparison.)
NMR:
Proton
Chemical Shift Table California State University, Stanislaus
Proton NMR Wizardfrom Steffan (St.) Thomas, University of Potsdam, Germany. A query based nmr tool in English and German. Useful in self-learning/testing applications. Supply a chemical shift value and a table of possibilities is produced with ranges of chemical shifts. Pages are cleanly designed and the built-in search engine works well.
13C Chemical Shift Calculator University of Potsdam, Germany, Produces13C spectra for phenyls, biphenyls, pyridines and pyridazines.
NMR Spectroscopy Problems On-Line:
![]()
![]() Prof.
Browne: University of Alberta
Prof.
Browne: University of Alberta
http://www.chem.ualberta.ca/~orglabs/spectroscopy/specmaster.html
![]() Prof. Merlic, UCLA, WebSpectra
Prof. Merlic, UCLA, WebSpectra
http://www.chem.ucla.edu/~webspectra/
![]() Prof. Smith, Notre
Dame
Prof. Smith, Notre
Dame
www.nd.edu/~smithgrp/structure/workbook.html
INTEGRATED ANALYSES:
Interpretation:
IR- example, 1H nmr - example,
MS - example, table 13C
nmr - example
Example compound:

Review the structural assignment of each of the on-line compounds reconciling any differences in earlier assignments and considering in toto: IR, MS
1H & 13C nmr data.
Apply IR data , MS data (provided by Dr. R.), and 1H & 13C nmr data to your individual unknown. NMR data generated using the department's 60 MHz FT-NMR spectrometer. Be sure that the structural assignment is complete and accurate.
Integrated Quiz/ Problems:
Spectroscopy for the Organic Chemistry Student from Professor Paul R. Young: University of Illinois, Chicago Organic Chemistry OnLine: Spectroscopy
-
Multiple Choice Quiz: Spectroscopy -----
Integrated Problems (Ten Problems}
Spectroscopy 4:
NMR Spectroscopy in Context (handouts)
1) Integrated Spectroscopy and Reaction Chemistry: Analysis of a Draft for Publication - pdf
----- Spectra - pdf
----- Team Form - pdf
"Is Peer Review Broken?", The Scientist, Volume 20 | Issue 2 | Page 26, (February 2006)
http://www.the-scientist.com/article/display/23061/
2) Dehydration of 1-ethyl-2-methylcyclohexanol: NMR application to determine the respective distribution of kinetic and thermodynamic products. See:
-
LABORATORY:
Diels Alder Reactions / Molecular Modeling
- Amino Acids: http://www.bio.cmu.edu/Courses/BiochemMols/AAViewer/AAVFrameset.htm
- Proteins: http://www.cryst.bbk.ac.uk/PPS2/course/index.html
- Trypsin musically: Man or Mouse? http://chemconnections.org/music/
- Cholinesterase: http://www.rcsb.org/pdb/molecules/pdb54_1.html
- Acetylcholinesterase: http://chemconnections.org/Presentations/Columbia/ace1.html
- Hemoglobin / Sickle Cell: http://chemconnections.org/Presentations/Columbia/slide8-3.html
- http://www.exploratorium.edu/cooking/bread/index.html
Introduction pdf
Synthesis Project: Electrophilic Aromatic Substitution / Friedel Crafts Acylation
1) Consult in-lab Team Project list for your Team members.
2) Refer to Team letter (A, B, C, etc.) for the list of starting aromatic compounds assigned to the Team.
3) If Team has less than 4 members, you may choose from the list which of those you will use. Read the project instructions. Select a starting material. Write up a pre-lab in lab notebook. Complete the Skills Check List for this experiment. Have pre-lab reviewed and initialed by Dr. R. before beginning. Complete the experiment.Project Instructions .pdf
Electrophilic Aromatic Substitution Worksheet .pdf
1H & 13C NMRs / Electrophilic Aromatic Substitution Worksheet .pdf
a)Nitration Quicktime Movie
b)Bromination Quicktime Movie
c)Acylation Quicktime Movie
REPORTS & Post lab Questions:
Provide a typed Cover Sheet with a title and the names of each Team member: Include a typed abstract describing the Team's overall results.
Include as attachments: individual typed Summary Reports for each product click here for an example:
1) Reactions and structures (Either drawn with a template [See Dr. R. if you plan this approach] or using ISIS Draw or Marvin; free/downloadable drawing programs), or similar program drawing program, ChemDraw, etc.
2) Concise experimental procedures. (Include % yield for crude product of undistilled samples only. Omit % Yield for distilled samples, but include boiling point for the sample that was spectroscopically analyzed. (If you did not obtain any product for analysis briefly explain why and include the NMR data from the following files for the product from your respective starting material. 1H 13C NMR fid files)
3) IR/NMR spectroscopy data,
4) Physical data: index of refraction
Attach copies of each team member’s raw lab research notebook pages to the respective typed individual summary reports. The notebook pages should be clearly and legibly written in the format that is described in the course syllabus to include title, name, date, etc. and include a short Results/Conclusions section. [Remember: Chemistry is fundamentally the study of matter.Your notebook's experimental record should include masses in grams for reactants and products, whether the product(s) were crude or distilled or not and whether or not % yield was calculated. If distilled, there should be masses and boiling ranges for each of the respective fractions.]5) Answer the following questions as a Team and turn in separately:
a) Name the major mono-nitration product of: 1) phenyl acetate, 2) 2,4-dinitrotoluene, 3) p-methoxybenzaldehyde
b) Beginning with benzene and 2,5-dichloro-2,5-dimethyl hexane, using any other necessary organic and inorganic reagents, outline a synthesis of versalide, which is a starting material for a class of aroma therapy compounds referred to as tetralin musks.
c) Find the boiling point for your individual product(s) from published sources. If you cannot find the exact structure select a compound that best approximates the product(s)' structure(s). Using a nomogram determine what boiling point or boiling range would be expected at 4 torr. Include this information in a table of all the products and include a reference where the boiling point was found.
d) Refer to the following list of criteria for "green chemistry". Identify those items in the list that relate to the reaction procedure that you just performed. For each item that you selected provide a recommendation for an improved alternative.
Green Chemistry: Science and Politics of Change
Martyn Poliakoff, J. Michael Fitzpatrick, Trevor R. Farren, Paul T. Anastas
Science, Volume 297, Number 5582, Issue of 2 Aug 2002, pp. 807-810.
Green Chemistry Principles
1. It is better to prevent waste than to treat or clean up waste after it is formed.
2. Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product.
3. Wherever practicable, synthetic methodologies should be designed to use and generate substances that possess little or no toxicity to human health and the environment.
4. Chemical products should be designed to preserve efficacy of function while reducing toxicity.
5. The use of auxiliary substances (e.g., solvents, separation agents, and so forth) should be made unnecessary wherever possible and innocuous when used.
6. Energy requirements should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods should be conducted at ambient temperature and pressure.
7. A raw material or feedstock should be renewable rather than depleting wherever technically and economically practicable.
8. Unnecessary derivatization (blocking group, protection/deprotection, temporary modification of physical/chemical processes) should be avoided whenever possible.
9. Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
10. Chemical products should be designed so that at the end of their function they do not persist in the environment and break down into innocuous degradation products.
11. Analytical methodologies need to be developed further to allow for real-time in-process monitoring and control before the formation of hazardous substances.
12. Substances and the form of a substance used in a chemical process should be chosen so as to minimize the potential for chemical accidents, including releases, explosions, and fireFollow-on:Analysis of experimental data from the products of three different experimental procedures that respectively used different Lewis acids: AlCl3, FeCl3 (orange + black), and FeCl3 (black).
Hydrolysis Rate of Esters in strong base: Click on the link and view the video. Work within your bioregulator teams following the procedure below. Turn in one completed lab form per group when due, consult the course calendar.
Procedure:
Place 2 mL of 50% ethanol, 2 drops of 1M NaOH, and 2 drops of universal indicator into each of 4 labeled test tubes. Stopper the tubes and shake each of them. Then, add 5 drops of one of the following esters: ethyl acetate, ethyl benzoate, ethyl butyrate, ethyl formate, to one of the labeled test tubes. Repeat for the remaining three esters and test tubes. Stpper and shake each until the ester dissolves. Record the color, and then heat the test tubes in a water bath to 50oC for a total of 30 minutes, recording the color every 10 minutes. Using a color chart convert the color to an approximate pH value.
See a similar mechanism to that used for the synthesis of dimedone. It is used in the preparation of oak moss odorant (an earthy odor): http://www.bojensen.net/Oakmoss/Oakmoss.htm (Courtesy of Bo Jensen)
It illustrates one method of synthesis for 2,4-dihydroxy-3,6-dimethylbenzoic acid methyl ester, the most important odour component in Oakmoss, Evernia prunastri (Usneaceae).
It has a very powerful odour: earthy, woody, like some lichens. It is used in perfumery in low concentrations.
References:
1) Sonn A, Berichte 1929;62B:3012
2) Eur.Pat. 133,960 (Chem.Abstr 1986;105:226057x)
3) Bauer K, Garbe D, Surburg H (1990) Common fragrance and flavor materials. Preparation, properties and uses. 2'nd rev. ed. VCH Germany.Synthesis of Dimedone
General Procedure:
Add ~25 mmol of dimethylmalonate to a dry round bottom flask equipped with a condenser. After the addition is complete place a drying tube on top of the condenser. Mix in ~26 mmol of a solution of 25% NaOCH3 in methanol. Add a spin bar or boiling chips, then heat the reaction mixture to just boiling in a water bath. Any solids should be dissolved at this point. Remove the water bath, from the top of the condenser slowly add ~26 mmol of mesityl oxide (freshly distilled) in small portions over a period of ~ 3 min. Reflux the reaction mixture for ~ 1hr. Evaporate the methanol until a moist solid residue is obtained.Add 20. mL of a 3 M solution of NaOH, reinstall the condenser and heat to reflux (90-95ºC) for ~45 min. Pour the reaction mixture with stirring into a beaker containing 15 mL of 6 M HCl. Carefully boil the mixture in the beaker under a hood until gas is no longer evolved. Cool the reaction mixture and collect the crystals by vacuum filtration. At this point, either dry the crystals or recrystallize them with a 50% acetone/water solution. Using either the crude or recrystallized product, obtain a melting point, IR, 1H NMR, and 13C NMR. Turn in the sample in a labeled vial. Answer the Postlab questions.
Postlab Questions:
a) Explain how IR could be used to determine the enol content.
b) Using the nmr spectrum for dimedone that is provided from the link below, calculate the percent of the enol present in the sample.
Dimedone NMR
c) Using your value in question b) calculate the equilibrium constant K.
d) Complete the in--lab/postlab SLO questionaire.
Preparation of Aldol Condensation Products

(Individual Assignments)
1) Prepare a prelab report in your lab notebook for your assigned reactants: the aldehyde and a ketone from the link above, using Procedure A, which follows.
2) This must be done independently. (Including both prelab and post-lab questions): Complete the prelab questions in the handout & turn-in.
3) Have the prelab notebook pages initialed before beginning.
Procedure A:
Place 2 mmol of ketone in a test tube or centrifuge tube and dissolve in ~ 4 mL of 95% ethanol. Add 8 mmol of aldehyde and 3 mL of 1 M NaOH. Let the reaction mixture stand at room temperature for ~15 minutes with occasional shaking. If little or no precipitation is observed, heat the reaction mixture in a boiling water bath until there is no further precipitation. Cool the mixture in an ice bath for ~5-10 minutes. Collect the solid by vacuum filtration. Wash the crystals with 2 mL of 4% acetic acid/95% ethanol v/v followed by 2 mL of cold 95% ethanol. Dry the product. Weigh the product. Calculate the % yield. Measure and record the melting point and IR spectrum of the product.Procedure B:
Place 0.006 moles of aldehyde, 0.003 moles of ketone, and 3 mL of 95% ethanol in a conical test tube. Mix until completely dissolved. Add 1 mL of 10% sodium hydroxide solution. Mix the contents until precipitation is observed. Let the mixture stand for another 20 minutes with occasional shaking. After the 20-minute period is completed, cool the mixture in an ice bath for 10 minutes. Using a Pasteur pipet, remove the liquid from the conical test tube, leaving the solid behind. In order to assure that no solid product is transferred out of the test tube, you could take a very small piece of cotton, and plug up the tip of the pipet (from the outside), such that only liquids are transferred into the pipet. Wash the crystals with 2 portions of 2 mL ice-cold water. Recrystallize the solid product in the same conical test tube, using 95% ethanol. Collect the product via vacuum filtration and allow it to dry. Note that you may need to tare your filter paper if you have very little product. Weigh your product. Measure the melting point and the IR spectrum of your obtained product.4) Post-lab Questions (Attach answers to notebook pages.)
Three unknown compounds with a molecular formula of C12H16O, (A, B, and C), were all optically active, all had strong IR bands in the 1700 cm–1 region. When A was treated with NaOD/D2O, three hydrogens were exchanged for deuterium, and the deuterated product of A remained optically active. When A was treated with I2/aq. NaOH, a yellow precipitate formed (A positive iodoform tests as in the prelab.) Compounds B and C did not give a yellow precipitate with I2/aq. NaOH. Compound B exchanged only one H for D when treated with NaOD/D2O, and the monodeuterated product of B was optically inactive. When compound C was reacted with NaOD/D2O, four H's were exchanged for D's, and the deuterated product of C remained optically active.
1) Give possible, plausible structures for A–C consistent with the preceding information. (There may be more than one possible structure for each of them.)
2) Which of your 3 structures would be the best candidate to produce a single product when reacted with benzaldehyde in an aldol condensaton? Briefly explain your answer. Draw a structure for the product of the condensation that would form at low temperature.
3) One aldehyde, trans-cinnamaldehyde was used in previous years, in 2009 the condensation did not occur. Spectroscopy Data for the "trans-cinnamaldehyde" from the bottle which was used in Spring 2009 was recorded. Provide a brief explanation of why the reaction did not occur.
Synthesis of Dimedone Derivatives
Literature Research / Organic Synthesis
A Remembrance to HeatherPART I: Calibrated Peer Review (CPR) on-line writing assignment
CPR UsernamesDesign and Development of Drugs
http://cpr.molsci.ucla.edu/1) You will read an article from the Journal of Chemical Education about organic synthesis and the history of many drugs and medicines,
K.C. Nicolaou, et. al., JChemEd, 75, 1226-1258, (1998); pdf files: Reading-1.pdf; Reading-2.pdf.
2) Learn about the way that one drug (aspirin) was discovered and how chemists contributed to its improvement.
3) Learn to identify new synthetic methods necessary in drug synthesis and future drug development.
4) Learn about a widely used approach to the rapid development of new chemical compounds using “Combinatorial Synthesis” and how “chemical libraries” (in a non-traditional sense) are used.
5) Write an essay on-line explaining how aspirin was developed, the methods chemists currently use to develop new and better drugs, which can be applied to any area including nano-materials, and in new applications of organic synthesis.
6) Review and appraise 3 of your classmates essays, and then reconsider and appraise your own submission.PART II: Literature Research
You are to select a compound which interests you. The accompanying list includes a number of compounds for your consideration, eg. thienamycin. Everyone will have a different individual compound. You are to identify and report your target compound, its CAS number, its General Class, eg. b-lactam antibiotics, and include a set of related Keywords, eg. thienamycin, b-lactam antibiotics, penems, carbopenems, monobactams, penicillins, etc., to Dr. R. by e-mail on or before April 16th. The compound does not have to come from the accompanying list, but you must have your selection approved before you begin your research.
Using Google Scholar, http://scholar.google.com/schhp?hl=en, Google Patents, http://www.google.com/patents?hl=en, and ERIC: Educational Resources Information Center, http://www.eric.ed.gov/, you are to find literature references, evaluate them, and produce a bibliography with abstracts that includes one or more relevant non-primary background reference(s) [books, review articles, etc.] or primary literature references [peer reviewed journals], which describe the General Class of compounds, their use, educational importance, and value to society, plus citing a minimum of 5 primary literature references, which describe the following topics: 1) physical, stereochemical and spectroscopic related data, e.g., [a], m.p or b.p., IR, 1H NMR, 13C NMR, 2) biological mode of action/ pharmacology/ toxicology, 3) one or more total or partial syntheses of the selected compound and/or its analogs. (More than one primary reference per topic is acceptable.)
Your report is to be type-written with a complete bibliography (6 references minimum: 1 non-primary, 5 primary), patents are acceptable as primary references, and it must include respective abstracts. See: Thienamycin Example.pdf. The report is to be type-written and include an introductory narrative section on the general class of compounds, their use, importance, and history. The report is to include the CAS number of your compound and a clearly drawn structure as a cover page with a Title, your name, and course and section information. You are to use a chemical drawing program such as ISIS/Draw or marvin/Draw for the drawing, which are free to students and faculty (See course Web site for download links.) or they can be used directly on the PS 110 computers. (Cutting and pasting, or freehand/ stenciled drawings are unacceptable.)
Two copies of the report are to be submitted by the deadline noted on the course calendar. Late reports will not be accepted.
Library Research / Organic Synthesis (Instructions)
A Remembrance to Heather
Library Research / Organic Synthesis
(Instructions)Calibrated Peer Review (CPR)
Design and Development of Drugs
http://cpr.molsci.ucla.edu/1) You are to read an article from the Journal of Chemical Education about organic synthesis and the history of many drugs and medicines,
K.C. Nicolaou, et. al., JChemEd, 75, 1226-1258, (1998); pdf files: Reading-1.pdf; Reading-2.pdf.
2) Learn about the way that one drug (aspirin) was discovered and how chemists contributed to its improvement.
3) Learn to identify new synthetic methods necessary in drug synthesis and future drug development.
4) Learn about a widely used approach to the rapid development of new chemical compounds using “Combinatorial Synthesis” and how “chemical libraries” (in a non-traditional sense) are used.
5) Write an essay explaining how aspirin was developed, the methods chemists currently use to develop new and better drugs, which can be applied to any area including nano-materials, and the future of organic synthesis.Library Research
DVC Library
http://www.dvc.edu/library/
DVC On-line Databases
http://www.dvc.edu/library/databases.htmUC Berkeley Chemistry Library & Maps: Campus, Zoom in, Chem Library
Chem Library Homepage: http://www.lib.berkeley.edu/CHEM/
March 2006, UC Berkeley Chem Library Calendar Hours
UCB Pathfinder
http://sunsite2.berkeley.edu:8000/
UC Davis
http://www.lib.ucdavis.edu/
http://www.lib.ucdavis.edu/pse/databases/index.html
University of Texas
http://www.lib.utexas.edu/Libs/Chem/info/
ERIC: Educational Resources Information Center
http://www.eric.ed.gov/
Examples of Some past papers:
Ibogaine
Leinamycin
Ascorbic Acid
Gibberellic Acid
Synthetic Challenges: Earn $$$ for your knowledge and imagination!
For example:
|
|
INNOCENTIVE 861088 Substituted Propionic Acid POSTED: APR 22, 2003 DEADLINE: JUL 30, 2003 $30,000 USD |
Insect Repellants: Synthesis of DEET

(1) Read: Lab Instructions
(2) Complete the synthetic procedure, purify a portion for IR analysis of your product. Answer the post lab questions at the end of the instructions, plus the following NMR related questions.
IR Fraction #1(bp <120 oC @ 2 torr)
Click for larger image.
IR Fraction #2 (bp 128-29 oC @ 2 torr)
Click for larger image.

1H NMR.fid Fraction #2 (bp 128-29 oC @ 2 torr)Composite Sample's 1H NMR.fid
Composite Sample's 13C NMR.fid
NMR Postlab Questions-10 .pdf
Extra Credit:
See: http://chemconnections.org/organic/chem226/Labs/Smell/ChemComm.htmlRead the article "Love Molecules". Identify the structure of the elephant pheromone and outline a synthesis of the compound starting from heptanoic acid lactone, 1-bromopentane, acetic anhydride and any other reagents that you choose.
(3) Enzymes & biological activity
Enzyme Docking (683K QuickTime Movie)
Enzyme catalyzed hydrolysis: Trypsin : p-nitrophenylacetate (498K, QuickTime Movie)
Separation and Identification of Unknown
High Resolution Masses for unknowns
Beynon Table:
http://www.chm.davidson.edu/java/beynon/beynon.html
Polymers: Slime and Gak (Silly Putty's Cousin)
Procedure .pdf
Report Form .pdf
Amino Acids/Proteins, Beads and the Chemistry of Flour:
Amino acids chart .pdf
Amino Acids Jmol/Rasmol Colors
Primary protein bead stringing
“The Chemistry of Cereals” and “Wheat, Flour and the Action of Water” .pdf
Protein Chemistry: Amino Acids, Flour, Dough & Elasticity .pdf
http://www.exploratorium.edu/cooking/bread/activity-gluten.html
http://members.lycos.nl/ClassoFoods/ukindex.htmlBread Recipes:
http://www.recipesource.com/baked-goods/breads/

 |
 |
- Carbohydrates: Worksheet
- Carbohydrates (University of Akron): http://ull.chemistry.uakron.edu/genobc/Chapter_17/
- Carbohydrate Active Enzymes: http://afmb.cnrs-mrs.fr/;
http://afmb.cnrs-mrs.fr/rubrique117.html
Bio-Recognition: Saccharides, Proteins, Influenza, SARS, HIV
See Professor Carolyn Bertozzi’s related LBL / You Tube Presentation:
http://www.youtube.com/watch?v=VBwNMR3C0Ys&feature=PlayList&p=10F61E434B646DE1&index=1 - Carbohydrate Active Enzymes: http://afmb.cnrs-mrs.fr/;
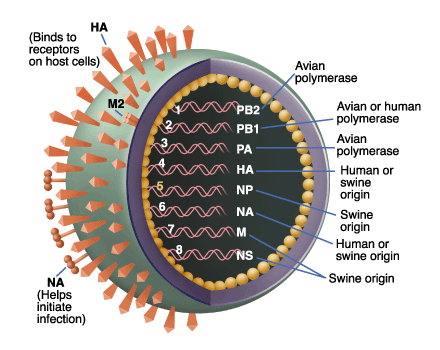
- The Actors
A Parent Virus Group (Hemagglutinin Proteins)
A Cell Membrane Group
A Cell Cytoplasm Group
A Viral Progeny Group
A Neuraminidase Group (Sialidase Enzyme)
An Anti-influenza Group (Neuraminidase Inhibitors)
The Play
Scene 1: The Parent Virus recognizes The Cell Membrane and infects the cell
Scene 2: Viral RNA is transported into the cell and cellular machinery in the Cell Cytoplasm builds new virions
Scene 3: The Viral Progeny escape from the cell only to clump together
Scene 4: Neuraminidase cleaves frees Viral Progeny from clumping
Alternate Ending:
Scene 4: The Neuraminidase inhibitor keeps Neuraminidase from freeing Viral Progeny
Click on the QuickTime links below the images for the 2013 Performances:
- 2013 AM Cast & Ensemble:

1) 2)
2) 3)
3)
- Flu Pandemic Monitoring: http://pandemicflu.gov/
- Flu Pandemics: http://chemconnections.org/ScanDbase/Flu-NYT-11-92.html
- SARS (Sudden Acute Respiratory Syndrone) http://www.who.int/csr/sars/en/
- Centers for Disease Control (CDC)
- http://www.promedmail.org
- http://www.nejm.com
- HIV
- HIV overcomes almost every antibody that attaches to its surface protein, gp120. The virus copies itself rapidly and mutates frequently, creating a staggering number of viral strains, which makes it a formidable challenge for antibodies to control. It has also evolved to effect the "primitive" innate immune system as well.
- HIV evolved to avoid 2 virus controlling enzymes in the innate immune system: Ref-1 & CEM 15
- Exercise to be done before doing the assigned reading:
- Color Wheel
- MC2 Java Applets: http://mc2.cchem.berkeley.edu/Java/
- Assigned Background Reading:
- Genetech's Access Excellence: http://www.gene.com/ae/AE/AEC/CC/vision_background.html Collected Chemistry & Biochemistry: Adapted from various sources, including ChemFinder and Metabolic Pathways, Genome Project (Japan) http://www.genome.ad.jp/kegg/metabolism_menu.html : http://chemconnections.llnl.gov/organic/Chem227/Vision/chem-vision.html and others.
- Functional Properties of Rhodopsin:http://www.isat.jmu.edu/users/klevicca/isat280/DETAILS.HTM
- You will need Chime, beta ver. 2.0, or RasMol to answer the following questions. The above image is retinal bound to rhodopsin, a vision protein that occurs in rod cells. How many helices and how many beta-sheets are found in rhoposin? Is the retinal in the all trans form or cis form? (Hint, the command: select hetero will select the retinal). Rhodopsin.PDB Name three enzymes related to"rhodopsin" that are also involved with rods and human vision. List the enzymes respective uv/visible absorbtion maxima and what color you would expect each of them to be? Postulate in molecular terms a brief explanation of the "experimental bird-watching" results noting probable changes to cellular enzymes in the cones that relate to your observed color changes and what the uv/visible absorbtion maxima of the enzymes might be?
- What structural features common to retinoids
and carotenes contribute to their importance in vision?
- Prelab pdf
- Experiment pdf
- Report
form pdf
Chiral Compounds and Green Chemistry: Reduction of a ketone by sodium borohydride and baker's yeast
Introduction .pdf
Procedure .pdf
Spectra .pdf:
1H & 13C NMRs, and IRs
Post Lab Questions.pdf

Carbohydrates, Glycoproteins and Related:
Viruses and influenza .pdf
Birds, Swine, Influenza & Us .pdf
"Why does the flu appear every year?" : A 4 act drama by Prof. Andy Mobley, Grinnell College
A Dramatic/Molecular Interpretation of the Influenza Virus's Life Cycle
Click on the links below the images for the 2010 Performances:
2010 Quicktime/AVI Movies:
M/W Production Team/59.5 Mb
T/Th Production Team/100.4Mb
2009 Quicktime/AVI Movies:
T/Th Production Team/65 Mb;
8 Mb
M/W Production Team/39.7 Mb;
161 Mb

Click on the above image for the 2003 Performance:
http://chemconnections.org/organic/chem227/Flu-slides/index.html

Click on the above image for the 2006 Performances:
Influenza 2006 Performances: Movie (17MB)

Synthesis of a bioregulator: 1-phenyl-3-(4-diethylaminoethoxyphenyl)-2-(E)-propen-1-one
Plant Hormones (phytohormones) are divided into five groups: cytokinins, abscisins, gibberellins, auxins, and ethylene. These groups interact and control the overall development of plant organs (eg. leaves, stems, roots, fruit) and effect plant behavior in relation to environmental conditions. Auxins control cell growth and are involved with plant functions such as phototropism (growing toward light), suppressing abscission (shedding: leaves, stalks, fruit, diseased parts), enhancing fruit production, and inhibiting growth. In this experiment you will prepare a synthetic auxin, which promotes the production of a carotenoid, lycopene. Carotenoids are highly conjugated and absorb ultraviolet radiation. They are colored and provide protection from sunlight and ultraviolet damage.
The synthesis involves two steps. The first is an aldol condensation of the enolate of acetophenone with p-hydroxybenzaldehyde. The resulting phenoxide that occurs in the reaction mixture is then directly used as a nucleophile and reacted with diethyl aminoethyl chloride.Care must be taken in the work up. See separation scheme.The bioassay of the product measures its effectiveness by determining the mass percent of lycopene produced by carotogenesis over a period of several days through the Beer’s Law analysis of the lycopene concentration.
Organize into a group of four. Determine the tasks that each group member will contribute to the overall experiment: in the synthesis: Parts A & B, and in the analysis & bioassay Parts: C & D. A one page typed summary will be submitted per group clearly highlighting the results from Parts B) and D).
The entire group is to answer all of the questions in the prelab form and prepare a lab notebook prelab for the reaction, bioassy, and analysis. Select one or more Team members to maintain notebook records for either the synthesis (Parts A & B), or spectral analysis (Part D), or bioassay (Part C), or combination of any of the three tasks including any calculations. These are to be attached to the typed summary cover sheet.

|

|
 
|

|

|
 |
 |
 |
|
|
|
Flatulence-I.pdf
Experimental Data / Questions:
Flatulence-II.pdf
Prelab form .pdf
Postlab form .pdf
Patented Invention: (Click image to see
patent.)

Click on each of the birds in the following table one at a time. When opened you will see a page with a colored bird on the left and an empty cage on the right. Focus your eyes on the bird's eye and slowly count to twenty; then quickly shift your view to the center of the empty cage. Record what you observe for each bird and then refer to the color wheel in the Web-link below. Write a brief description relating your results to the color wheel, comparing pairs of colors and their relative locations on the wheel. Also refer to the MC2 emission and absorption spectrum simulation tools to vary colors. These can be linked from the MC2 Java Applets page.
Exercise adapted from the Exploratorium's Snacks: http://www.exploratorium.edu/snacks/bird_in_cage.html
Questions:
How many different types of cone cells are there in the eye that respond to color?
What do you think the color of these cells might be if they were viewable to you?
Based on the experiment done in class,sketch the eye and illustrate where on the retina these cells are located.
|
|
Wave length (nm) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Human Genome:
-
http://www.wellcome.ac.uk/en/genome/index.html
| 1 GCA17 CUC33 GUG | 49 GAU |
| 2 CCC18 CUA34 AAU | 50 GAC |
| 3 GUU19 UUU35 AAC | 51 CGU |
| 4 CUU20 CUG36 CAG | 52 UAG |
| 5 UUG21 UAU37 UGU | 53 AAG |
| 6 AUG22 UAC38 CGC | 54 UGG |
| 7 CCU23 UAA39 GGU | 55 AGG |
| 8 GCC24 AAA40 GGC | 56 GAG |
| 9 GCU25 UGA41 GGA | 57 GAA |
| 10 UCG26 AGA42 GGG | 58 CCG |
| 11 AUA27 AGC43 CGA | 59 UCC |
| 12 UUA28 AGU44 CGG | 60 ACC |
| 13 UUC29 GCG45 UGC | 61 ACU |
| 14 AUC30 CCA46 CAA | 62 UCU |
| 15 UCA31 GUC47 CAC | 63 AUU |
| 16 ACG32 GUA48 CAU | 64 ACA |
(2003)
SARS: Genetic translation
1 HLKNGTCGLVELEKGVLPQLEQPYVFIKRSDALSTNHGHKVVELVAEMDGIQYGRSGI
2 TLGVLVPHVGETPIAYRNVLLRKNGNKGAGGHSYGIDLKSYDLGDELGTDPIEDYEQN
3 WNTKHGSGALRELTRELNGGAVTRYVDNNFCGPDGYPLDCIKDFLARAGKSMCTLSEQ
4 LDYIESKRGVYCCRDHEHEIAWFTERSDKSYEHQTPFEIKSAKKFDTFKGECPKFVFP
5 LNSKVKVIQPRVEKKKTEGFMGRIRSVYPVASPQECNNMHLSTLMKCNHCDEVSWQTC
6 DFLKATCEHCGTENLVIEGPTTCGYLPTNAVVKMPCPACQDPEIGPEHSVADYHNHSN
7 IETRLRKGGRTRCFGGCVFAYVGCYNKRAYWVPRASADIGSGHTGITGDNVETLNEDL
8 LEILSRERVNINIVGDFHLNEEVAIILASFSASTSAFIDTIKSLDYKSFKTIVESCGN
9 YKVTKGKPVKGAWNIGQQRSVLTPLCGFPSQAAGVIRSIFARTLDAANHSIPDLQRAA
10 VTILDGISEQSLRLVDAMVYTSDLLTNSVIIMAYVTGGLVQQTSQWLSNLLGTTVEKL
11 RPIFEWIEAKLSAGVEFLKDAWEILKFLITGVFDIVKGQIQVASDNIKDCVKCFIDVV
12 NKALEMCIDQVTIAGAKLRSLNLGEVFIAQSKGLYRQCIRGKEQLQLLMPLKAPKEVT
13 FLEGDSHDTVLTSEEVVLKNGELEALETPVDSFTNGAIVGTPVCVNGLMLLEIKDKEQ
14 YCALSPGLLATNNVFRLKGGAPIKGVTFGEDTVWEVQGYKNVRITFELDERVDKVLNE
15 KCSVYTVESGTEVTEFACVVAEAVVKTLQPVSDLLTNMGIDLDEWSVATFYLFDDAGE
16 ENFSSRMYCSFYPPDEEEEDDAECEEEEIDETCEHEYGTEDDYQGLPLEFGASAETVR
17 VEEEEEEDWLDDTTEQSEIEPEPEPTPEEPVNQFTGYLKLTDNVAIKCVDIVKEAQSA
18 NPMVIVNAANIHLKHGGGVAGALNKATNGAMQKESDDYIKLNGPLTVGGSCLLSGHNL
19 AKKCLHVVGPNLNAGEDIQLLKAAYENFNSQDILLAPLLSAGIFGAKPLQSLQVCVQT
20 VRTQVYIAVNDKALYEQVVMDYLDNLKPRVEAPKQEEPPNTEDSKTEEKSVVQKPVDV
21 KPKIKACIDEVTTTLEETKFLTNKLLLFADINGKLYHDSQNMLRGEDMSFLEKDAPYM
22 VGDVITSGDITCVVIPSKKAGGTTEMLSRALKKVPVDEYITTYPGQGCAGYTLEEAKT
(2005-2006)
Will Bird Flu mutate and transfer from human to human? Where
did SARS go?
(April 2009)
Will SwineFlu become pandemic nightmare?
High resolution MS Molecular ion data
End
of Course Survey: (Anonymous survey)